The Prophylactic Protection of Salmonella Typhimurium Infection by Lentilactobacillus buchneri GX0328-6 in Mice
- PMID: 37668855
- PMCID: PMC11573835
- DOI: 10.1007/s12602-023-10145-8
The Prophylactic Protection of Salmonella Typhimurium Infection by Lentilactobacillus buchneri GX0328-6 in Mice
Abstract
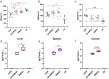
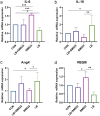
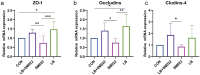
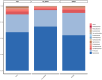
Salmonellosis is a disease caused by non-typhoid Salmonella, and although some lactic acid bacteria strains have been shown previously to relieve Salmonellosis symptoms, little has been studied about the preventive mechanism of Lentilactobacillus buchneri (L. buchneri) against Salmonella infection in vivo. Therefore, the L. buchneri was fed to C57BL/6 mice for 10 days to build a protective system of mice to study its prevention and possible mechanisms. The results showed that L. buchneri GX0328-6 alleviated symptoms caused by Salmonella typhimurium infection among C57BL/6 mice, including low survival rate, weight loss, increase in immune organ index and hepatosplenomegaly, and modulated serum immunoglobulin levels and intrinsic immunity. Importantly, the L. buchneri GX0328-6 enhanced the mucosal barrier of the mouse jejunum by upregulating the expression of tight junction proteins such as ZO-1, occludins, and claudins-4 and improved absorptive capacity by increasing the length of mouse jejunal villus and the ratio of villus length to crypt depth and decreasing the crypt depth. L. buchneri GX0328-6 reduced the intestinal proliferation and invasion of Salmonella typhimurium by modulating the expression of antimicrobial peptides in the intestinal tract of mice, and reduced intestinal inflammation and systemic spread in mice by downregulating the expression of IL-6 and promoting the expression of IL-10. Furthermore, L. buchneri GX0328-6 increased the relative abundance of beneficial bacteria colonies and decreased the relative abundance of harmful bacteria in the cecum microflora by modulating the microflora in the cecum contents.
Keywords: Lentilactobacillus buchneri; Salmonella typhimurium; Gene expression; Gut microbiota.
© 2023. The Author(s).
Conflict of interest statement
Figures




Similar articles
-
Daily intake of household-produced milk kefir on Salmonella Typhimurium infection in C57BL/6 mice: mortality, microbiota modulation, and immunological implications.J Appl Microbiol. 2024 Nov 4;135(11):lxae249. doi: 10.1093/jambio/lxae249. J Appl Microbiol. 2024. PMID: 39317667
-
Antimicrobial peptide AP2 ameliorates Salmonella Typhimurium infection by modulating gut microbiota.BMC Microbiol. 2025 Feb 5;25(1):64. doi: 10.1186/s12866-025-03776-0. BMC Microbiol. 2025. PMID: 39910418 Free PMC article.
-
Anti-infective mechanisms induced by a probiotic Lactobacillus strain against Salmonella enterica serovar Typhimurium infection.Int J Food Microbiol. 2010 Apr 15;138(3):223-31. doi: 10.1016/j.ijfoodmicro.2010.01.020. Epub 2010 Feb 1. Int J Food Microbiol. 2010. PMID: 20193971
-
Innate immune response to Salmonella typhimurium, a model enteric pathogen.Gut Microbes. 2012 Mar-Apr;3(2):62-70. doi: 10.4161/gmic.19141. Epub 2012 Mar 1. Gut Microbes. 2012. PMID: 22198618 Free PMC article. Review.
-
[Salmonellosis: distribution of the causative organism in the body].Zh Mikrobiol Epidemiol Immunobiol. 1984 Oct;(10):3-6. Zh Mikrobiol Epidemiol Immunobiol. 1984. PMID: 6151767 Review. Russian. No abstract available.
Cited by
-
Evaluation of probiotic properties and complete genome analysis of lactic acid bacteria isolated from crested ibis Nipponia nippon feces.Front Microbiol. 2025 Apr 9;16:1552264. doi: 10.3389/fmicb.2025.1552264. eCollection 2025. Front Microbiol. 2025. PMID: 40270811 Free PMC article.
References
-
- Fantasia M, Filetici E (1994) Salmonella enteritidis in Italy. Int J Food Microbiol 21(1–2):7–13. 10.1016/0168-1605(94)90194-5 - PubMed
-
- Omwandho C, Kubota T (2010) Salmonella enterica serovar Enteritidis: a mini-review of contamination routes and limitations to effective control. Japan Agricultural Research Quarterly 44(1):7–16
-
- Giansanti F, Giardi MF, Botti D (2006) Avian cytokines–an overview. Curr Pharm Des 12(24):3083–3099. 10.2174/138161206777947542 - PubMed
-
- Won G, Lee JH (2017) Salmonella Typhimurium, the major causative agent of foodborne illness inactivated by a phage lysis system provides effective protection against lethal challenge by induction of robust cell-mediated immune responses and activation of dendritic cells. Vet Res 48(1):66. 10.1186/s13567-017-0474-x - PMC - PubMed
MeSH terms
Grants and funding
- AB21238003, AB21220005-4/the Guangxi Key R&D Program
- 2021GXNSFAA196058/the Natural Science Foundation of Guangxi Province
- CARS-42-55/the National Technical System Construction Project for Waterfowl Industry
- nycytxgxcxtd/the Guangxi Broiler Industry Innovation Team Construction Project
- 202118/the Major Science and Technology Project of Liangqing District
LinkOut - more resources
Full Text Sources

