Rapid Immunodot AQP4 Assay for Neuromyelitis Optica Spectrum Disorder
- PMID: 37669037
- PMCID: PMC10481325
- DOI: 10.1001/jamaneurol.2023.2974
Rapid Immunodot AQP4 Assay for Neuromyelitis Optica Spectrum Disorder
Abstract
Importance: Immunoglobulin G autoantibodies for aquaporin 4 (AQP4-IgG) serve as diagnostic biomarkers for neuromyelitis optica spectrum disorder (NMOSD), and the most sensitive and specific laboratory tests for their detection are cell-based assays (CBAs). Nevertheless, the limited availability of special instruments limits the widespread use of CBAs in routine laboratories.
Objective: To validate an enzyme immunodot assay for simple and rapid detection of AQP4-IgG.
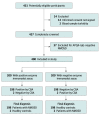
Design, setting, and participants: This multicenter case-control study, conducted from May 2020 to February 2023, involved 4 medical centers (3 in China and 1 in Korea). The study included patients with AQP4-IgG-positive NMOSD, patients with other immune-related diseases, and healthy control individuals. Participants were excluded if they did not agree to participate or if their serum sample had turbidity.
Exposures: Serum AQP4 antibodies measured with immunodot assay.
Main outcomes and measures: The main outcome was performance of the immunodot assay compared with the gold standard CBA for detecting AQP4-IgG. To examine generalizability, cross-validation in Korea and at a second site in China, validation of patients with other immune-related diseases, and follow-up validation of the original cohort were performed.
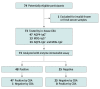
Results: A total of 836 serum samples were collected; 400 were included in the diagnostic study and 436 in the validation sets. In a head-to-head diagnostic study involving 200 patients with NMOSD with AQP4-IgG (mean [SD] age, 43.1 [13.5] years; 188 [94%] female) and 200 healthy controls, use of an immunodot assay demonstrated antibody detection performance comparable to that of the gold standard (κ = 98.0%). The validation sets included 47 patients with NMOSD and 26 patients with other autoimmune diseases from Korea, 31 patients with NMOSD at a second site in China, 275 patients with other diseases, and 57 patients with NMOSD at follow-up. In the validation study, of 436 cases, 2 (<1%) were false positive and none were false negative. The CBA identified 332 AQP4-IgG-positive samples and 504 negative samples (200 [40%] in controls and 304 [60%] in patients with other diseases); 2 of the positive cases (<1%) were false negative and 4 of the negative cases (<1%) were false positive. The overall sensitivity of the immunodot assay was 99.4% (95% CI, 97.8%-99.9%), and the specificity was 99.2% (95% CI, 98.0%-99.8%).
Conclusions and relevance: This case-control study found that the immunodot assay was comparable to CBA for detecting AQP4-IgG. With its time- and cost-efficient characteristics, the immunodot assay may be a practical option for AQP4-IgG detection.
Conflict of interest statement
Figures

Comment in
-
Rapid AQP4-IgG detection in neuromyelitis optica spectrum disorder.Nat Rev Neurol. 2023 Nov;19(11):638. doi: 10.1038/s41582-023-00882-3. Nat Rev Neurol. 2023. PMID: 37740136 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

