Tregs integrate native and CAR-mediated costimulatory signals for control of allograft rejection
- PMID: 37669115
- PMCID: PMC10619441
- DOI: 10.1172/jci.insight.167215
Tregs integrate native and CAR-mediated costimulatory signals for control of allograft rejection
Abstract
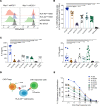
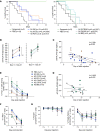
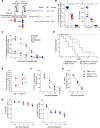
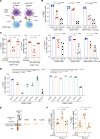
Tregs expressing chimeric antigen receptors (CAR-Tregs) are a promising tool to promote transplant tolerance. The relationship between CAR structure and Treg function was studied in xenogeneic, immunodeficient mice, revealing advantages of CD28-encoding CARs. However, these models could underrepresent interactions between CAR-Tregs, antigen-presenting cells (APCs), and donor-specific Abs. We generated Tregs expressing HLA-A2-specific CARs with different costimulatory domains and compared their function in vitro and in vivo using an immunocompetent model of transplantation. In vitro, the CD28-encoding CAR had superior antigen-specific suppression, proliferation, and cytokine production. In contrast, in vivo, Tregs expressing CARs encoding CD28, ICOS, programmed cell death 1, and GITR, but not 4-1BB or OX40, all extended skin allograft survival. To reconcile in vitro and in vivo data, we analyzed effects of a CAR encoding CD3ζ but no costimulatory domain. These data revealed that exogenous costimulation from APCs can compensate for the lack of a CAR-encoded CD28 domain. Thus, Tregs expressing a CAR with or without CD28 are functionally equivalent in vivo, mediating similar extension of skin allograft survival and controlling the generation of anti-HLA-A2 alloantibodies. This study reveals a dimension of CAR-Treg biology and has important implications for the design of CARs for clinical use in Tregs.
Keywords: Costimulation; Immunology; Immunotherapy; Tolerance; Transplantation.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

