Novel progestogenic androgens for male contraception: design, synthesis, and activity of C7 α-substituted testosterone†
- PMID: 37669128
- PMCID: PMC10724455
- DOI: 10.1093/biolre/ioad111
Novel progestogenic androgens for male contraception: design, synthesis, and activity of C7 α-substituted testosterone†
Abstract
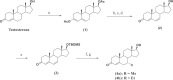
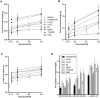
Male contraceptive development has included use of testosterone (T) with or without a progestin or the use of a single molecule such as progestogenic androgens (PA) for suppression of testicular T production. Expanding upon the vast amount of data accumulated from nortestosterone (NT), NT analogs, and their prodrugs, a new series of PA, the C7 methyl, and ethyl α-substituted T analogs 7α-Methyltestosterone (7α-MT) and 7α-Ethyltestosterone (7α-ET), respectively, were hypothesized and designed to have superior androgenic and progestogenic activities when compared with parent T. Results from androgen receptor and progesterone receptor competitive binding and transcriptional activation assays showed favorable activities for these T analogs. Additionally, 7α-MT and 7α-ET were shown to be active substrates for aromatase in vitro, mitigating a potential negative impact on bone mineral density with long-term use. In conjunction with this observation, the diminished metabolism of these T analogs by 5α-reductase may reduce potential concerns for prostatic growth. In the Hershberger in vivo rat bioassay, 7α-MT and 7α-ET showed superior androgenic and anabolic activities as compared with T. These C7 α-substituted T analogs also showed clear progestogenic activity in the McPhail bioassay which evaluated endometrial glandular arborization in a rabbit model. The discovery of aromatizable molecules with reduced metabolism by 5α-reductase that have androgenic, anabolic, and progestogenic properties indicates that the core and/or prodrugs of 7α-MT and 7α-ET are promising molecules for further development as male contraceptive PAs.
Keywords: male contraception; metabolism; progestogenic androgen; testosterone analogs.
Published by Oxford University Press on behalf of Society for the Study of Reproduction 2023.
Conflict of interest statement
The authors report no conflict of interest.
Figures





References
-
- Brady BM, Amory JK, Perheentupa A, Zitzmann M, Hay CJ, Apter D, Anderson RA, Bremner WJ, Pollanen P, Nieschlag E, Wu FCW, Kersemaekers WM. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod 2006; 21:285–294. - PubMed
-
- Mommers E, Kersemaekers WM, Elliesen JR, Kepers M, Apter D, Behre HM, Beynon J, Bouloux PM, Costantino A, Gerbershagen H-P, Grønlund L, Heger-Mahn D, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metabol 2008; 93:2572–2580. - PubMed
-
- Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI, Meriggiola MC, Misro MM, Noe G, Wu FCW, Festin MPR, Habib NA, et al. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metabol 2016; 101:4779–4788. - PubMed
-
- Kamischke A, Heuermann T, Krüger K, Von Eckardstein S, Schellschmidt I, Rübig A, Nieschlag E. An effective hormonal male contraceptive using testosterone Undecanoate with oral or injectable Norethisterone preparations. J Clin Endocrinol Metabol 2002; 87:530–539. - PubMed
-
- Meriggiola MC, Costantino A, Saad F, D’Emidio L, Morselli Labate AM, Bertaccini A, Bremner WJ, Rudolph I, Ernst M, Kirsch B, Martorana G, Pelusi G. Norethisterone Enanthate plus testosterone Undecanoate for male contraception: effects of various injection intervals on spermatogenesis, reproductive hormones, testis, and prostate. J Clin Endocrinol Metabol 2005; 90:2005–2014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

