Exploring the relationship between patient-relevant outcomes and Alzheimer's disease progression assessed using the clinical dementia rating scale: a systematic literature review
- PMID: 37669257
- PMCID: PMC10470645
- DOI: 10.3389/fneur.2023.1208802
Exploring the relationship between patient-relevant outcomes and Alzheimer's disease progression assessed using the clinical dementia rating scale: a systematic literature review
Abstract
Background: People with Alzheimer's disease (AD) have difficulties in performing activities of daily living (ADLs) as the disease progresses, commonly experience neuropsychiatric symptoms (NPS), and often have comorbidities such as cardiovascular disease. These factors all contribute to a requirement for care and considerable healthcare costs in AD. The Clinical Dementia Rating (CDR) scale is a widely used measure of dementia staging, but the correlations between scores on this scale and patient-/care partner-relevant outcomes have not been characterized fully. We conducted a systematic literature review to address this evidence gap.
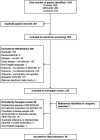
Methods: Embase, MEDLINE, and the Cochrane Library were searched September 13, 2022, to identify published studies (no restriction by date or country) in populations with mild cognitive impairment due to AD or AD dementia. Studies of interest reported data on the relationships between CDR Global or CDR-Sum of Boxes (CDR-SB) scores and outcomes including NPS, comorbidities, ADLs, nursing home placement, healthcare costs, and resource use.
Results: Overall, 58 studies met the inclusion criteria (42 focusing on comorbidities, 14 on ADLs or dependence, five on nursing home placement, and six on economic outcomes). CDR/CDR-SB scores were correlated with the frequency of multiple NPS and with total scores on the Neuropsychiatric Inventory. For cardiovascular comorbidities, no single risk factor was consistently linked to AD progression. Increasing CDR/CDR-SB scores were correlated with decline in multiple different measures of ADLs and were also associated with nursing home placement and increasing costs of care.
Conclusion: NPS, ADLs, and costs of care are clearly linked to AD progression, as measured using CDR Global or CDR-SB scores, from the earliest stages of disease. This indicates that scores derived from the CDR are a meaningful way to describe the severity and burden of AD for patients and care partners across disease stages.
Keywords: Alzheimer’s disease; activities of daily living; clinical dementia rating (CDR); comorbidity; healthcare costs; healthcare resource use; neuropsychiatric inventory (NPI); nursing home placement.
Copyright © 2023 Cummings, Hahn-Pedersen, Eichinger, Freeman, Clark, Tarazona and Lanctôt.
Conflict of interest statement
KL has acted as an advisor/consultant for BioXcel Therapeutics, Bright Minds Biosciences, Cerevel Therapeutics, Eisai, GW Pharmaceuticals, IGCPharma, Kondor Pharma, Lundbeck, Merck, Novo Nordisk A/S, Praxis Precision Medicines, and Sumitomo Pharma. JH-P, AC, and LT are employed by Novo Nordisk A/S, which funded the systematic literature review. They contributed to design of the systematic literature review, data interpretation, review and revision of the manuscript, and approval for publication. CE and CF are employed by Oxford PharmaGenesis, which received funding for conducting the systematic literature review. JC has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Biogen, Cassava, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lilly, LSP, Merck, NervGen, Novo Nordisk A/S, Oligomerix, Ono, Otsuka, PRODEO, Prothena, ReMYND, Resverlogix, Roche, Signant Health, Suven, and United Neuroscience pharmaceutical, assessment, and investment companies. He is supported by US National Institute of General Medical Sciences (NIGMS) grant P20GM109025; National Institute of Neurological Disorders and Stroke (NINDS) grant U01NS093334; National Institute on Aging (NIA) grants R01AG053798, P20AG068053, P30AG072959, and R35AG71476; the Alzheimer’s Disease Drug Discovery Foundation (ADDF); the Ted and Maria Quirk Endowment; and the Joy Chambers-Grundy Endowment.
Figures






Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Donepezil for dementia due to Alzheimer's disease.Cochrane Database Syst Rev. 2018 Jun 18;6(6):CD001190. doi: 10.1002/14651858.CD001190.pub3. Cochrane Database Syst Rev. 2018. PMID: 29923184 Free PMC article.
-
Pharmacotherapies for sleep disturbances in Alzheimer's disease.Cochrane Database Syst Rev. 2014 Mar 21;(3):CD009178. doi: 10.1002/14651858.CD009178.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Nov 16;11:CD009178. doi: 10.1002/14651858.CD009178.pub3. PMID: 24659320 Updated.
-
Vitamin E for Alzheimer's dementia and mild cognitive impairment.Cochrane Database Syst Rev. 2017 Apr 18;4(4):CD002854. doi: 10.1002/14651858.CD002854.pub5. Cochrane Database Syst Rev. 2017. PMID: 28418065 Free PMC article.
-
Vitamin E for Alzheimer's dementia and mild cognitive impairment.Cochrane Database Syst Rev. 2017 Jan 27;1(1):CD002854. doi: 10.1002/14651858.CD002854.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Apr 18;4:CD002854. doi: 10.1002/14651858.CD002854.pub5. PMID: 28128435 Free PMC article. Updated.
Cited by
-
Family physicians' perspectives on outcomes, processes, and policies in dementia care.Health Aff Sch. 2025 Jan 13;3(1):qxae167. doi: 10.1093/haschl/qxae167. eCollection 2025 Jan. Health Aff Sch. 2025. PMID: 39811073 Free PMC article.
-
Low serum serotonin is associated with functional decline, mild behavioural impairment and brain atrophy in dementia-free subjects.Brain Commun. 2025 Jan 9;7(1):fcaf005. doi: 10.1093/braincomms/fcaf005. eCollection 2025. Brain Commun. 2025. PMID: 39816197 Free PMC article.
-
The translational power of Alzheimer's-based organoid models in personalized medicine: an integrated biological and digital approach embodying patient clinical history.Front Cell Neurosci. 2025 May 15;19:1553642. doi: 10.3389/fncel.2025.1553642. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40443709 Free PMC article. Review.
-
Longitudinal trajectories of cognitive, functional, and neuropsychiatric decline in Alzheimer's disease during COVID-19 lockdown in South Korea.Sci Rep. 2025 Mar 8;15(1):8081. doi: 10.1038/s41598-025-92497-5. Sci Rep. 2025. PMID: 40057581 Free PMC article.
-
Online Interventions for Family Carers of People with Dementia That Focus on Support Strategies for Daily Living: A Mixed Methods Systematic Review.Behav Sci (Basel). 2025 Jun 25;15(7):863. doi: 10.3390/bs15070863. Behav Sci (Basel). 2025. PMID: 40723647 Free PMC article. Review.
References
-
- Alzheimer’s Disease International . Global estimates of informal care. (2018). Available at: www.alzint.org/u/global-estimates-of-informal-care.pdf [Accessed December 23, 2022].
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

