Cis P-tau is a central circulating and placental etiologic driver and therapeutic target of preeclampsia
- PMID: 37669931
- PMCID: PMC10480164
- DOI: 10.1038/s41467-023-41144-6
Cis P-tau is a central circulating and placental etiologic driver and therapeutic target of preeclampsia
Abstract
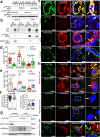
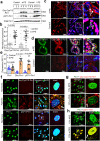
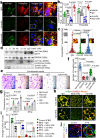
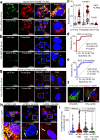
Preeclampsia (PE) is the leading cause of maternal and fetal mortality globally and may trigger dementia later in life in mothers and their offspring. However, the etiological drivers remain elusive. Cis P-tau is an early etiological driver and blood biomarker in pre-clinical Alzheimer's and after vascular or traumatic brain injury, which can be targeted by stereo-specific antibody, with clinical trials ongoing. Here we find significant cis P-tau in the placenta and serum of PE patients, and in primary human trophoblasts exposed to hypoxia or sera from PE patients due to Pin1 inactivation. Depletion of cis P-tau from PE patient sera by the antibody prevents their ability to disrupt trophoblast invasion and endovascular activity and to cause the PE-like pathological and clinical features in pregnant humanized tau mice. Our studies uncover that cis P-tau is a central circulating etiological driver and its stereo-specific antibody is valuable for early PE diagnosis and treatment.
© 2023. Springer Nature Limited.
Conflict of interest statement
X.Z.Z. and K.P.L. are inventors of issued patents and/or pending patent applications including on
Figures
Similar articles
-
TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review.
-
Effects of sirtuin 1 deficiency on trophoblasts and its implications in the pathogenesis of pre-eclampsia.J Obstet Gynaecol. 2023 Dec;43(2):2282103. doi: 10.1080/01443615.2023.2282103. Epub 2023 Nov 15. J Obstet Gynaecol. 2023. PMID: 37966393
-
Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia.Autophagy. 2020 Oct;16(10):1771-1785. doi: 10.1080/15548627.2019.1707494. Epub 2019 Dec 26. Autophagy. 2020. PMID: 31856641 Free PMC article.
-
Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat.Mol Hum Reprod. 2017 Jul 1;23(7):509-519. doi: 10.1093/molehr/gax024. Mol Hum Reprod. 2017. PMID: 28402512
-
The role of the Pin1-cis P-tau axis in the development and treatment of vascular contribution to cognitive impairment and dementia and preeclampsia.Front Cell Dev Biol. 2024 Apr 2;12:1343962. doi: 10.3389/fcell.2024.1343962. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38628595 Free PMC article. Review.
Cited by
-
Protein Misfolding in Pregnancy: Current Insights, Potential Mechanisms, and Implications for the Pathogenesis of Preeclampsia.Molecules. 2024 Jan 26;29(3):610. doi: 10.3390/molecules29030610. Molecules. 2024. PMID: 38338354 Free PMC article. Review.
-
Novel Role of Pin1-Cis P-Tau-ApoE Axis in the Pathogenesis of Preeclampsia and Its Connection with Dementia.Biomedicines. 2024 Dec 26;13(1):29. doi: 10.3390/biomedicines13010029. Biomedicines. 2024. PMID: 39857613 Free PMC article. Review.
-
ORF3a is a key driver of maternal SARS-CoV-2 infection-associated placental dysfunction.Res Sq [Preprint]. 2025 Jul 3:rs.3.rs-6857689. doi: 10.21203/rs.3.rs-6857689/v1. Res Sq. 2025. PMID: 40630515 Free PMC article. Preprint.
-
SARS-CoV-2 ORF3a Protein Impairs Syncytiotrophoblast Maturation, Alters ZO-1 Localization, and Shifts Autophagic Pathways in Trophoblast Cells and 3D Organoids.bioRxiv [Preprint]. 2024 Sep 25:2024.09.25.614931. doi: 10.1101/2024.09.25.614931. bioRxiv. 2024. PMID: 39386577 Free PMC article. Preprint.
-
Exploiting sweet relief for preeclampsia by targeting autophagy-lysosomal machinery and proteinopathy.Exp Mol Med. 2024 May;56(5):1206-1220. doi: 10.1038/s12276-024-01234-x. Epub 2024 May 17. Exp Mol Med. 2024. PMID: 38760513 Free PMC article.
References
-
- Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398:341–354. - PubMed
-
- Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. - PubMed
-
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

