Subsets of preoperative sex hormones in testicular germ cell cancer: a retrospective multicenter study
- PMID: 37669975
- PMCID: PMC10480169
- DOI: 10.1038/s41598-023-41915-7
Subsets of preoperative sex hormones in testicular germ cell cancer: a retrospective multicenter study
Abstract
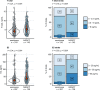
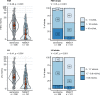
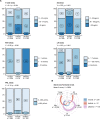
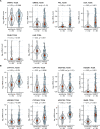
Preoperative homeostasis of sex hormones in testicular germ cell tumor (TGCT) patients is scarcely characterized. We aimed to explore regulation of sex hormones and their implications for histopathological parameters and prognosis in TGCT using a data-driven explorative approach. Pre-surgery serum concentrations of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (T), estradiol (E2) and prolactin were measured in a retrospective multicenter TGCT cohort (n = 518). Clusters of patients were defined by latent class analysis. Clinical, pathologic and survival parameters were compared between the clusters by statistical hypothesis testing, Random Forest modeling and Peto-Peto test. Cancer tissue expression of sex hormone-related genes was explored in the publicly available TCGA cohort (n = 149). We included 354 patients with pure seminoma and 164 patients with non-seminomatous germ cell tumors (NSGCT), with a median age of 36 years. Three hormonal clusters were defined: 'neutral' (n = 228) with normal sex hormone homeostasis, 'testicle' (n = 91) with elevated T and E2, low pituitary hormones, and finally 'pituitary' subset (n = 103) with increased FSH and LH paralleled by low-to-normal levels of the gonadal hormones. Relapse-free survival in the hormonal subsets was comparable (p = 0.64). Cancer tissue expression of luteinizing hormone- and follicle-stimulating hormone-coding genes was significantly higher in seminomas, while genes of T and E2 biosynthesis enzymes were strongly upregulated in NSGCT. Substantial percentages of TGCT patients are at increased risk of sex hormone dysfunction at primary diagnosis before orchiectomy. TGCT may directly influence systemic hormonal homeostasis by in-situ synthesis of sex hormones.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dieckmann KP, Simonsen-Richter H, Kulejewski M, et al. Serum tumour markers in testicular germ cell tumours: Frequencies of elevated levels and extents of marker elevation are significantly associated with clinical parameters and with response to treatment. Biomed. Res. Int. 2019;2019:5030349. doi: 10.1155/2019/5030349. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

