Placental growth factor exerts a dual function for cardiomyogenesis and vasculogenesis during heart development
- PMID: 37669989
- PMCID: PMC10480216
- DOI: 10.1038/s41467-023-41305-7
Placental growth factor exerts a dual function for cardiomyogenesis and vasculogenesis during heart development
Erratum in
-
Author Correction: Placental growth factor exerts a dual function for cardiomyogenesis and vasculogenesis during heart development.Nat Commun. 2024 Jan 4;15(1):283. doi: 10.1038/s41467-023-44507-1. Nat Commun. 2024. PMID: 38177121 Free PMC article. No abstract available.
Abstract
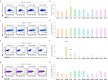
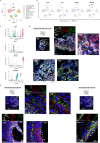
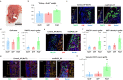
Cardiogenic growth factors play important roles in heart development. Placental growth factor (PLGF) has previously been reported to have angiogenic effects; however, its potential role in cardiogenesis has not yet been determined. We analyze single-cell RNA-sequencing data derived from human and primate embryonic hearts and find PLGF shows a biphasic expression pattern, as it is expressed specifically on ISL1+ second heart field progenitors at an earlier stage and on vascular smooth muscle cells (SMCs) and endothelial cells (ECs) at later stages. Using chemically modified mRNAs (modRNAs), we generate a panel of cardiogenic growth factors and test their effects on enhancing cardiomyocyte (CM) and EC induction during different stages of human embryonic stem cell (hESC) differentiations. We discover that only the application of PLGF modRNA at early time points of hESC-CM differentiation can increase both CM and EC production. Conversely, genetic deletion of PLGF reduces generation of CMs, SMCs and ECs in vitro. We also confirm in vivo beneficial effects of PLGF modRNA for development of human heart progenitor-derived cardiac muscle grafts on murine kidney capsules. Further, we identify the previously unrecognized PLGF-related transcriptional networks driven by EOMES and SOX17. These results shed light on the dual cardiomyogenic and vasculogenic effects of PLGF during heart development.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare the following competing interests that K.R.C. is a past co-founder of Moderna Therapeutics and is currently a member of the board of directors of eTheRNA. All other authors declare no competing interests.
Figures




References
-
- Madonna R, et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016;37:1789–1798. doi: 10.1093/eurheartj/ehw113. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

