Quantitative detection of formaldehyde using solid phase microextraction gas chromatography-mass spectrometry coupled to cysteamine scavenging
- PMID: 37670131
- PMCID: PMC10480157
- DOI: 10.1038/s41598-023-41609-0
Quantitative detection of formaldehyde using solid phase microextraction gas chromatography-mass spectrometry coupled to cysteamine scavenging
Abstract
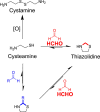
Formaldehyde (HCHO) is a toxic and carcinogenic pollutant and human metabolite that reacts with biomolecules under physiological conditions. Quantifying HCHO is essential for ongoing biological and biomedical research on HCHO; however, its reactivity, small size and volatility make this challenging. Here, we report a novel HCHO detection/quantification method that couples cysteamine-mediated HCHO scavenging with SPME GC-MS analysis. Our NMR studies confirm cysteamine as an efficient and selective HCHO scavenger that out-competes O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine, the most commonly used scavenger, and forms a stable thiazolidine amenable to GC-MS quantification. Validation of our GC-MS method using FDA and EMA guidelines revealed detection and quantification limits in the nanomolar and micromolar ranges respectively, while analysis of bacterial cell lysate confirmed its applicability in biological samples. Overall, our studies confirm that cysteamine scavenging coupled to SPME GC-MS analysis provides a sensitive and chemically robust method to quantify HCHO in biological samples.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kamps JJAG, Hopkinson RJ, Schofield CJ, Claridge TDW. How formaldehyde reacts with amino acids. Commun. Chem. 2019;2:1–14. doi: 10.1038/s42004-019-0224-2. - DOI
-
- Yan M, et al. Emission of volatile organic compounds from new furniture products and its impact on human health. Hum. Ecol. Risk Assess. 2019;25:1886–1906. doi: 10.1080/10807039.2018.1476126. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

