Refractory post-surgical cystoid macular edema managed following suprachoroidal microcatheterization and delivery of triamcinolone
- PMID: 37670276
- PMCID: PMC10478372
- DOI: 10.1186/s12886-023-03110-0
Refractory post-surgical cystoid macular edema managed following suprachoroidal microcatheterization and delivery of triamcinolone
Abstract
Background: Post-surgical macular edema (ME) is a common cause of prolonged visual impairment. Here we report on the feasibility and clinical outcomes from the use of a novel suprachoroidal microcatheter to treat post-surgical chronic ME by the posterior suprachoroidal placement of a triamcinolone acetonide (TA) suspension.
Methods: Two patients were catheterized with the Oxulumis suprachoroidal delivery system on two separate occasions starting 5 and 10 mm posterior to the limbus. The catheter only remains in the suprachoroidal space for the time of the drug administration. Visual acuity and spectral domain optical coherence tomography (SD-OCT) changes were followed over several weeks to months to determine the duration of ME resolution.
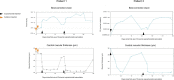
Results: Suprachoroidal microcatheterization for posterior delivery of triamcinolone was possible in all attempts using the illuminated Oxulumis catheter. No reflux, scleral or choroidal trauma was observed. There was no intraocular pressure rise during the follow-up period. The triamcinolone deposit was visible on infrared imaging and on SD-OCT a choroidal elevation was visible. Both progressively disappeared over time. A rapid resolution of ME associated with improved vision was observed following each injection for 3 to 7 months with a TA dose of 2.4 mg or 4 mg.
Conclusions: In these patients with poorly responsive ME, posterior suprachoroidal TA led to a visible suprachoroidal drug deposit and prolonged visual improvement. The Oxulumis microcatheterization device performed as expected and was not associated with any complications.
Keywords: CME; Drug visualization; Infrared; Macular edema; Microcatheterization; OCT; Posterior; Steroid; Suprachoroidal.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
MdS is a consultant for Oxular; FA and RY are employees of Oxular Ltd; MG has no financial disclosures.
Figures




Similar articles
-
Suprachoroidal Space Triamcinolone Acetonide: A Review in Uveitic Macular Edema.Drugs. 2022 Sep;82(13):1403-1410. doi: 10.1007/s40265-022-01763-7. Epub 2022 Aug 26. Drugs. 2022. PMID: 36018461 Free PMC article. Review.
-
Effect of Suprachoroidal Triamcinolone Acetonide on Intraocular Pressure in Macular Edema: A Retrospective Study.Cureus. 2025 Jan 11;17(1):e77282. doi: 10.7759/cureus.77282. eCollection 2025 Jan. Cureus. 2025. PMID: 39931603 Free PMC article.
-
Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial.Ophthalmology. 2020 Jul;127(7):948-955. doi: 10.1016/j.ophtha.2020.01.006. Epub 2020 Jan 10. Ophthalmology. 2020. PMID: 32173113 Clinical Trial.
-
SUPRACHOROIDAL INJECTION OF TRIAMCINOLONE ACETONIDE, CLS-TA, FOR MACULAR EDEMA DUE TO NONINFECTIOUS UVEITIS: A Randomized, Phase 2 Study (DOGWOOD).Retina. 2019 Oct;39(10):1880-1888. doi: 10.1097/IAE.0000000000002279. Retina. 2019. PMID: 30113933 Clinical Trial.
-
Suprachoroidal Triamcinolone Acetonide Injection to Treat Macular Edema: A Review.J Vitreoretin Dis. 2024 Oct 17:24741264241275271. doi: 10.1177/24741264241275271. Online ahead of print. J Vitreoretin Dis. 2024. PMID: 39539834 Free PMC article. Review.
References
-
- de Smet MD, Okada AA. Cystoid macular edema in uveitis. Dev Ophthal. 2010;47:136–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

