Single-Center Experience in Vaccination of Children in Special Risk Groups: A Multidisciplinary Institutional Consensus Protocol
- PMID: 37670552
- PMCID: PMC10544037
- DOI: 10.5152/TurkArchPediatr.2023.23097
Single-Center Experience in Vaccination of Children in Special Risk Groups: A Multidisciplinary Institutional Consensus Protocol
Abstract
Objective: Despite marked improvements in the accessibility of childhood vaccines, knowledge gaps remain about the vaccination of children in special risk groups (SRG). This study aimed to analyze the clinical data of children vaccinated in SRG in a single-center unit to contribute to the clinical evidence for the specific planning of immunization of children in SRG. The second- ary aim is to present institutional consensus on the vaccination of children in SRG.
Materials and methods: This retrospective study was conducted at a single-center pediatric vaccination clinic. Patient charts between 2018 and 2021 were retrospectively reviewed, and clinical and laboratory data were extracted. Serial joint meetings with multiple healthcare pro- fessionals were performed to develop an institutional protocol for vaccination.
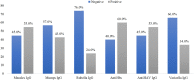
Results: There were 479 children vaccinated between 2018 and 2021 for reasons such as post- chemotherapy, after hematopoietic stem cell transplantation, before/after solid organ trans- plantation, allergies, and chronic diseases. Of these, 298 (62.2%) children vaccinated in the unit due to a history of food or vaccine allergies were excluded. One hundred eighty-one children were vaccinated at a median age of 11 [7-15] years. Most children were vaccinated after treat- ment for malignancies. Solid tumors were the most frequent malignancy (67%), followed by acute lymphoblastic leukemia (29.0%) and acute myeloid leukemia (4.0%). Institutional vacci- nation protocols for cancer survivors, hematopoietic stem cells, and solid organ recipient chil- dren were developed and presented.
Conclusion: There is a need to prepare national guidelines for vaccinating children with altered immunocompetence. Sharing vaccination practices by multidisciplinary vaccination units might increase and provide knowledge to develop national policies.
Figures
References
-
- Available at: https://dosyasb.saglik.gov.tr/Eklenti/1117,gbpgenelge2008pdf.pdf?0. Accessed July 02, 2023.
LinkOut - more resources
Full Text Sources