Comparative analysis of spike-specific IgG Fc glycoprofiles elicited by adenoviral, mRNA, and protein-based SARS-CoV-2 vaccines
- PMID: 37670790
- PMCID: PMC10475480
- DOI: 10.1016/j.isci.2023.107619
Comparative analysis of spike-specific IgG Fc glycoprofiles elicited by adenoviral, mRNA, and protein-based SARS-CoV-2 vaccines
Abstract
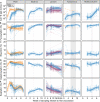
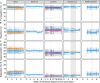
IgG antibodies are important mediators of vaccine-induced immunity through complement- and Fc receptor-dependent effector functions. Both are influenced by the composition of the conserved N-linked glycan located in the IgG Fc domain. Here, we compared the anti-Spike (S) IgG1 Fc glycosylation profiles in response to mRNA, adenoviral, and protein-based COVID-19 vaccines by mass spectrometry (MS). All vaccines induced a transient increase of antigen-specific IgG1 Fc galactosylation and sialylation. An initial, transient increase of afucosylated IgG was induced by membrane-encoding S protein formulations. A fucose-sensitive ELISA for antigen-specific IgG (FEASI) exploiting FcγRIIIa affinity for afucosylated IgG was used as an orthogonal method to confirm the LC-MS-based afucosylation readout. Our data suggest that vaccine-induced anti-S IgG glycosylation is dynamic, and although variation is seen between different vaccine platforms and individuals, the evolution of glycosylation patterns display marked overlaps.
Keywords: Glycomics; Immune response; Immunology; Microbiology.
© 2023 The Author(s).
Conflict of interest statement
M.L.G. and R.R. are employees of Janssen Pharmaceuticals and M.L.G. is a shareholder in Johnson & Johnson. A.S. and W.A.d.J. are employees at AdaptVac, a company commercializing virus-like particle display technology and vaccines, including several patents. A.S., A.S., T.G.T., and M.N. are founders of AdaptVac and listed as coinventors on a patent covering the AP205 CLP vaccine platform technology (WO2016112921 A1) licensed to AdaptVac. Janssen Pharmaceuticals sponsored IgG glycosylation analysis at LUMC. Sanquin provided consultancy services to Janssen Pharmaceuticals during this study. All other authors declare they have no conflicts of interests.
Figures


References
-
- Adjobimey T., Meyer J., Sollberg L., Bawolt M., Berens C., Kovačević P., Trudić A., Parcina M., Hoerauf A. Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 Vaccines. Front. Immunol. 2022;13:917905. - PMC - PubMed
-
- Solforosi L., Kuipers H., Jongeneelen M., Rosendahl Huber S.K., van der Lubbe J.E.M., Dekking L., Czapska-Casey D.N., Izquierdo Gil A., Baert M.R.M., Drijver J., et al. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J. Exp. Med. 2021;218:e20202756. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

