Unravelling the diversity in water usage among wild banana species in response to vapour pressure deficit
- PMID: 37670859
- PMCID: PMC10475999
- DOI: 10.3389/fpls.2023.1068191
Unravelling the diversity in water usage among wild banana species in response to vapour pressure deficit
Abstract
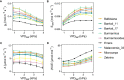
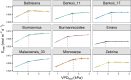
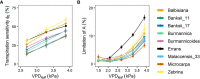
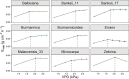
The rise in global temperature is not only affecting plant functioning directly, but is also increasing air vapour pressure deficit (VPD). The yield of banana is heavily affected by water deficit but so far breeding programs have never addressed the issue of water deficit caused by high VPD. A reduction in transpiration at high VPD has been suggested as a key drought tolerance breeding trait to avoid excessive water loss, hydraulic failure and to increase water use efficiency. In this study, stomatal and transpiration responses under increasing VPD at the leaf and whole-plant level of 8 wild banana (sub)species were evaluated, displaying significant differences in stomatal reactivity. Three different phenotypic groups were identified under increasing VPD. While (sub)species of group III maintained high transpiration rates under increasing VPD, M. acuminata ssp. errans (group I), M. acuminata ssp. zebrina (group II) and M. balbisiana (group II) showed the highest transpiration rate limitations to increasing VPD. In contrast to group I, group II only showed strong reductions at high VPD levels, limiting the cost of reduced photosynthesis and strongly increasing their water use efficiency. M. acuminata ssp. zebrina and M. balbisiana thus show the most favourable responses. This study provides a basis for the identification of potential parent material in gene banks for breeding future-proof bananas that cope better with lack of water.
Keywords: breeding; drought tolerance; stomatal conductance; transpiration; vapour pressure deficit; water use efficiency; wild banana species.
Copyright © 2023 Eyland, Gambart, Swennen and Carpentier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
The effect of blue light on stomatal oscillations and leaf turgor pressure in banana leaves.Plant Cell Environ. 2017 Jul;40(7):1143-1152. doi: 10.1111/pce.12907. Epub 2017 Mar 30. Plant Cell Environ. 2017. PMID: 28098339
-
Gravimetric phenotyping of whole plant transpiration responses to atmospheric vapour pressure deficit identifies genotypic variation in water use efficiency.Plant Sci. 2016 Oct;251:101-109. doi: 10.1016/j.plantsci.2016.05.018. Epub 2016 May 28. Plant Sci. 2016. PMID: 27593468
-
Understanding and Exploiting Transpiration Response to Vapor Pressure Deficit for Water Limited Environments.Front Plant Sci. 2022 May 10;13:893994. doi: 10.3389/fpls.2022.893994. eCollection 2022. Front Plant Sci. 2022. PMID: 35620701 Free PMC article.
-
Plant responses to rising vapor pressure deficit.New Phytol. 2020 Jun;226(6):1550-1566. doi: 10.1111/nph.16485. Epub 2020 Mar 20. New Phytol. 2020. PMID: 32064613 Review.
-
Systemic effects of rising atmospheric vapor pressure deficit on plant physiology and productivity.Glob Chang Biol. 2021 May;27(9):1704-1720. doi: 10.1111/gcb.15548. Epub 2021 Mar 8. Glob Chang Biol. 2021. PMID: 33683792 Free PMC article. Review.
References
-
- Aubert B., Catsky J. (1970). The onset of photosynthetic CO2 influx in banana leaf segments as related to stomatal diffusion resistance at different air humidities. Photosynthetica 4 (3), 254–256.
LinkOut - more resources
Full Text Sources

