Involvement of metalloproteinase and nitric oxide synthase/nitric oxide mechanisms in early decidual angiogenesis-vascularization of normal and experimental pathological mouse placenta related to maternal alcohol exposure
- PMID: 37670932
- PMCID: PMC10476144
- DOI: 10.3389/fcell.2023.1207671
Involvement of metalloproteinase and nitric oxide synthase/nitric oxide mechanisms in early decidual angiogenesis-vascularization of normal and experimental pathological mouse placenta related to maternal alcohol exposure
Abstract
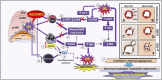
Successful pregnancy for optimal fetal growth requires adequate early angiogenesis and remodeling of decidual spiral arterioles during placentation. Prior to the initiation of invasion and endothelial replacement by trophoblasts, interactions between decidual stromal cells and maternal leukocytes, such as uterine natural killer cells and macrophages, play crucial roles in the processes of early maternal vascularization, such as proliferation, apoptosis, migration, differentiation, and matrix and vessel remodeling. These placental angiogenic events are highly dependent on the coordination of several mechanisms at the early maternal-fetal interface, and one of them is the expression and activity of matrix metalloproteinases (MMPs) and endothelial nitric oxide synthases (NOSs). Inadequate balances of MMPs and nitric oxide (NO) are involved in several placentopathies and pregnancy complications. Since alcohol consumption during gestation can affect fetal growth associated with abnormal placental development, recently, we showed, in a mouse model, that perigestational alcohol consumption up to organogenesis induces fetal malformations related to deficient growth and vascular morphogenesis of the placenta at term. In this review, we summarize the current knowledge of the early processes of maternal vascularization that lead to the formation of the definitive placenta and the roles of angiogenic MMP and NOS/NO mechanisms during normal and altered early gestation in mice. Then, we propose hypothetical defective decidual cellular and MMP and NOS/NO mechanisms involved in abnormal decidual vascularization induced by perigestational alcohol consumption in an experimental mouse model. This review highlights the important roles of decidual cells and their MMP and NOS balances in the physiological and pathophysiological early maternal angiogenesis-vascularization during placentation in mice.
Keywords: MMPs; NO; alcohol consumption; maternal angiogenesis; mouse; placental vascularization.
Copyright © 2023 Gualdoni, Barril, Jacobo, Pacheco Rodríguez and Cebral.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Early Abnormal Placentation and Evidence of Vascular Endothelial Growth Factor System Dysregulation at the Feto-Maternal Interface After Periconceptional Alcohol Consumption.Front Physiol. 2022 Feb 2;12:815760. doi: 10.3389/fphys.2021.815760. eCollection 2021. Front Physiol. 2022. PMID: 35185604 Free PMC article. Review.
-
Perigestational alcohol consumption induces altered early placentation and organogenic embryo growth restriction by disruption of trophoblast angiogenic factors.Reprod Biomed Online. 2021 Mar;42(3):481-504. doi: 10.1016/j.rbmo.2020.10.015. Epub 2020 Nov 2. Reprod Biomed Online. 2021. PMID: 33549483
-
Decidual vascularization during organogenesis after perigestational alcohol ingestion.Reproduction. 2019 Jul;158(1):109-122. doi: 10.1530/REP-18-0230. Reproduction. 2019. PMID: 31042673
-
Cellular and molecular oxidative stress-related effects in uterine myometrial and trophoblast-decidual tissues after perigestational alcohol intake up to early mouse organogenesis.Mol Cell Biochem. 2018 Mar;440(1-2):89-104. doi: 10.1007/s11010-017-3158-y. Epub 2017 Aug 18. Mol Cell Biochem. 2018. PMID: 28822072
-
Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes.Am J Obstet Gynecol. 2019 Nov;221(5):437-456. doi: 10.1016/j.ajog.2019.05.044. Epub 2019 Jun 1. Am J Obstet Gynecol. 2019. PMID: 31163132 Review.
References
-
- Alexander C. M., Hansell E. J., Behrendtsen O., Flannery M. L., Kishnani N. S., Hawkes S. P., et al. (1996). Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development 122, 1723–1736. 10.1242/dev.122.6.1723 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

