Histone 4 lysine 20 tri-methylation: a key epigenetic regulator in chromatin structure and disease
- PMID: 37671044
- PMCID: PMC10475950
- DOI: 10.3389/fgene.2023.1243395
Histone 4 lysine 20 tri-methylation: a key epigenetic regulator in chromatin structure and disease
Abstract
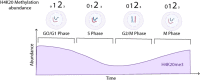
Chromatin is a vital and dynamic structure that is carefully regulated to maintain proper cell homeostasis. A great deal of this regulation is dependent on histone proteins which have the ability to be dynamically modified on their tails via various post-translational modifications (PTMs). While multiple histone PTMs are studied and often work in concert to facilitate gene expression, here we focus on the tri-methylation of histone H4 on lysine 20 (H4K20me3) and its function in chromatin structure, cell cycle, DNA repair, and development. The recent studies evaluated in this review have shed light on how H4K20me3 is established and regulated by various interacting partners and how H4K20me3 and the proteins that interact with this PTM are involved in various diseases. Through analyzing the current literature on H4K20me3 function and regulation, we aim to summarize this knowledge and highlights gaps that remain in the field.
Keywords: H4K20me3; cancer; chromatin; disease; heterochromatin; histone; homeostasis; methylation.
Copyright © 2023 Agredo and Kasinski.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

