Differences in eConsent among diagnosis groups
- PMID: 37671244
- PMCID: PMC10475468
- DOI: 10.1016/j.conctc.2023.101200
Differences in eConsent among diagnosis groups
Abstract
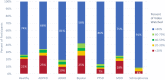
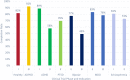
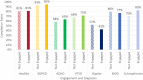
eConsent is an electronic informed consent experience that contains videos, word flags, and knowledge checks, in addition to an electronic version of the informed consent document to enhance clinical trial participants' understanding of what they are consenting to. There are numerous perceived benefits of eConsent, however despite these benefits, adoption has remained low. eConsent data from 27 clinical trials was analyzed to gain insights and understand differences in the consenting process between diagnosis groups. It was found that those with bipolar disorder spent significantly less time on the instructional video while those with schizophrenia spent significantly more. Participants with autosomal dominant polycystic kidney disease (ADPKD) had the lowest engagement while participants with schizophrenia were the most engaged. Knowledge check scores for participants with attention deficit hyperactivity disorder (ADHD) were significantly lower than the other diagnosis groups. The data available through eConsent provides crucial insights into the consenting differences among participants' diagnoses. Understanding these differences will support tailoring the eConsent process to a more patient centric design and ensure study participants understand what they are consenting to.
Keywords: Clinical trial; Consent; Informed consent; Patient centricity; eConsent.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Deborah Profit, William Carson, Candace Saldarini, and Hannah Glenny are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Leonard Chuck is an employee of Diablo Clinical Research, Inc. This research was supported by Otsuka Pharmaceutical Development & Commercialization, Inc.
Figures
References
-
- CISCRP . 2019. 2019 Perceptions and Insights Study: Participation Experience.https://www.ciscrp.org/wp-content/uploads/2019/12/Participation-Experien...
-
- Transcelerate . TransCelerate BioPharma, Inc; 2017. eConsent: Implementation Guidance.https://www.transceleratebiopharmainc.com/wp-content/uploads/2017/10/eCo...
-
- Cascade E. Electronic informed consent: The star bench-warmer? Center Watch. 2019. https://www.centerwatch.com/articles/14004
LinkOut - more resources
Full Text Sources

