Live-imaging studies reveal how microclots and the associated inflammatory response enhance cancer cell extravasation
- PMID: 37671502
- PMCID: PMC10561694
- DOI: 10.1242/jcs.261225
Live-imaging studies reveal how microclots and the associated inflammatory response enhance cancer cell extravasation
Abstract
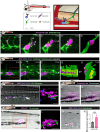
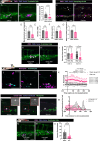
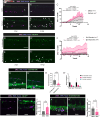
Previous clinical studies and work in mouse models have indicated that platelets and microclots might enable the recruitment of immune cells to the pre-metastatic cancer niche, leading to efficacious extravasation of cancer cells through the vessel wall. Here, we investigated the interaction between platelets, endothelial cells, inflammatory cells, and engrafted human and zebrafish cancer cells by live-imaging studies in translucent zebrafish larvae, and show how clotting (and clot resolution) act as foci and as triggers for extravasation. Fluorescent tagging in each lineage revealed their dynamic behaviour and potential roles in these events, and we tested function by genetic and drug knockdown of the contributing players. Morpholino knockdown of fibrinogen subunit α (fga) and warfarin treatment to inhibit clotting both abrogated extravasation of cancer cells. The inflammatory phenotype appeared fundamental, and we show that forcing a pro-inflammatory, tnfa-positive phenotype is inhibitory to extravasation of cancer cells.
Keywords: Cancer; Coagulation; Inflammation; Zebrafish.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Visualizing extravasation dynamics of metastatic tumor cells.J Cell Sci. 2010 Jul 1;123(Pt 13):2332-41. doi: 10.1242/jcs.069443. Epub 2010 Jun 8. J Cell Sci. 2010. PMID: 20530574 Free PMC article.
-
Venous Thrombosis and Thrombocyte Activity in Zebrafish Models of Quantitative and Qualitative Fibrinogen Disorders.Int J Mol Sci. 2021 Jan 11;22(2):655. doi: 10.3390/ijms22020655. Int J Mol Sci. 2021. PMID: 33440782 Free PMC article.
-
Chemical Modulators of Fibrinogen Production and Their Impact on Venous Thrombosis.Thromb Haemost. 2021 Apr;121(4):433-448. doi: 10.1055/s-0040-1718414. Epub 2020 Dec 10. Thromb Haemost. 2021. PMID: 33302304 Free PMC article.
-
Inflammatory Responses during Tumour Initiation: From Zebrafish Transgenic Models of Cancer to Evidence from Mouse and Man.Cells. 2020 Apr 20;9(4):1018. doi: 10.3390/cells9041018. Cells. 2020. PMID: 32325966 Free PMC article. Review.
-
Possible involvement of cytokines in diffuse intravascular coagulation and thrombosis.Baillieres Best Pract Res Clin Haematol. 1999 Sep;12(3):343-59. doi: 10.1053/beha.1999.0029. Baillieres Best Pract Res Clin Haematol. 1999. PMID: 10856974 Review.
Cited by
-
The role and regulation of integrins in cell migration and invasion.Nat Rev Mol Cell Biol. 2025 Feb;26(2):147-167. doi: 10.1038/s41580-024-00777-1. Epub 2024 Sep 30. Nat Rev Mol Cell Biol. 2025. PMID: 39349749 Review.
-
Reprogramming macrophages with R848-loaded artificial protocells to modulate skin and skeletal wound healing.J Cell Sci. 2024 Aug 15;137(16):jcs262202. doi: 10.1242/jcs.262202. Epub 2024 Aug 29. J Cell Sci. 2024. PMID: 39078119 Free PMC article.
-
Compromised Blood-Brain Barrier Junctions Enhance Melanoma Cell Intercalation and Extravasation.Cancers (Basel). 2023 Oct 20;15(20):5071. doi: 10.3390/cancers15205071. Cancers (Basel). 2023. PMID: 37894438 Free PMC article.
References
-
- Anfray, C., Mainini, F., Digifico, E., Maeda, A., Sironi, M., Erreni, M., Anselmo, A., Ummarino, A., Gandoy, S., Expósito, F.et al. (2021). Intratumoral combination therapy with poly(I:C) and resiquimod synergistically triggers tumor-associated macrophages for effective systemic antitumoral immunity. J. Immunother. Cancer 9, e002408. 10.1136/jitc-2021-002408 - DOI - PMC - PubMed
-
- Best, M. G., Sol, N., In ‘t Veld, S. G. J. G., Vancura, A., Muller, M., Niemeijer, A.-L. N., Fejes, A. V., Tjon Kon Fat, L.-A., Huis In ‘t Veld, A. E., Leurs, C.et al. (2017). Swarm intelligence-enhanced detection of non-small-cell lung cancer using tumor-educated platelets. Cancer Cell 32, 238-252.e239. 10.1016/j.ccell.2017.07.004 - DOI - PMC - PubMed
-
- Borrego, I., Frobert, A., Ajalbert, G., Valentin, J., Kaltenrieder, C., Fellay, B., Stumpe, M., Cook, S., Dengjel, J. and Giraud, M.-N. (2022). Fibrin, bone marrow cells and macrophages interactively modulate cardiomyoblast fate. Biomedicines 10, 527. 10.3390/biomedicines10030527 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

