Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction
- PMID: 37671592
- PMCID: PMC10628503
- DOI: 10.1093/humrep/dead177
Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction
Abstract
Study question: Are human ovarian aging and the age-related female fertility decline caused by oxidative stress and mitochondrial dysfunction in oocytes?
Summary answer: We found oxidative damage in oocytes of advanced maternal age, even at the primordial follicle stage, and confirmed mitochondrial dysfunction in such oocytes, which likely resulted in the use of alternative energy sources.
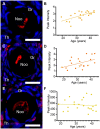
What is known already: Signs of reactive oxygen species-induced damage and mitochondrial dysfunction have been observed in maturing follicles, and even in early stages of embryogenesis. However, although recent evidence indicates that also primordial follicles have metabolically active mitochondria, it is still often assumed that these follicles avoid oxidative phosphorylation to prevent oxidative damage in dictyate arrested oocytes. Data on the influence of ovarian aging on oocyte metabolism and mitochondrial function are still limited.
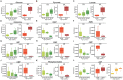
Study design, size, duration: A set of 39 formalin-fixed and paraffin-embedded ovarian tissue biopsies were divided into different age groups and used for immunofluorescence analysis of oxidative phosphorylation activity and oxidative damage to proteins, lipids, and DNA. Additionally, 150 immature oocytes (90 germinal vesicle oocytes and 60 metaphase I oocytes) and 15 cumulus cell samples were divided into different age groups and used for targeted metabolomics and lipidomics analysis.
Participants/materials, setting, methods: Ovarian tissues used for immunofluorescence microscopy were collected through PALGA, the nationwide network, and registry of histo- and cytopathology in The Netherlands. Comprehensive metabolomics and lipidomics were performed by liquid-liquid extraction and full-scan mass spectrometry, using oocytes and cumulus cells of women undergoing ICSI treatment based on male or tubal factor infertility, or fertility preservation for non-medical reasons.
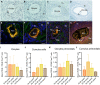
Main results and the role of chance: Immunofluorescence imaging on human ovarian tissue indicated oxidative damage by protein and lipid (per)oxidation already at the primordial follicle stage. Metabolomics and lipidomics analysis of oocytes and cumulus cells in advanced maternal-age groups demonstrated a shift in the glutathione-to-oxiglutathione ratio and depletion of phospholipids. Age-related changes in polar metabolites suggested a decrease in mitochondrial function, as demonstrated by NAD+, purine, and pyrimidine depletion, while glycolysis substrates and glutamine accumulated, with age. Oocytes from women of advanced maternal age appeared to use alternative energy sources like glycolysis and the adenosine salvage pathway, and possibly ATP which showed increased production in cumulus cells.
Limitations, reasons for caution: The immature oocytes used in this study were all subjected to ovarian stimulation with high doses of follicle-stimulating hormones, which might have concealed some age-related differences.
Wider implications of the findings: Further studies on how to improve mitochondrial function, or lower oxidative damage, in oocytes from women of advanced maternal age, for instance by supplementation of NAD+ precursors to promote mitochondrial biogenesis, are warranted. In addition, supplementing the embryo medium of advanced maternal-age embryos with such compounds could be a treatment option worth exploring.
Study funding/competing interest(s): The study was funded by the Amsterdam UMC. The authors declare to have no competing interests.
Trial registration number: N/A.
Keywords: NAD+; mitochondrial dysfunction; oocyte quality; ovarian ageing; oxidative damage.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare to have no competing interests.
Figures


References
-
- Ahmed TA, Ahmed SM, El-Gammal Z, Shouman S, Ahmed A, Mansour R, El-Badri N.. Oocyte aging: the role of cellular and environmental factors and impact on female fertility. Adv Exp Med Biol 2019. - PubMed
-
- Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 2014;68:475–478. - PubMed
-
- Bouet PE, Boueilh T, de la Barca JMC, Boucret L, Blanchard S, Ferré-L'Hotellier V, Jeannin P, Descamps P, Procaccio V, Reynier P. et al. The cytokine profile of follicular fluid changes during ovarian ageing. J Gynecol Obstet Hum Reprod 2020;49:101704. - PubMed
-
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szöllösi MS, Debey P.. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod 1999;60:580–587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

