Homodimeric Granzyme A Opsonizes Mycobacterium tuberculosis and Inhibits Its Intracellular Growth in Human Monocytes via Toll-Like Receptor 4 and CD14
- PMID: 37671668
- PMCID: PMC10938207
- DOI: 10.1093/infdis/jiad378
Homodimeric Granzyme A Opsonizes Mycobacterium tuberculosis and Inhibits Its Intracellular Growth in Human Monocytes via Toll-Like Receptor 4 and CD14
Abstract
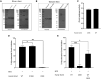
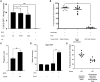
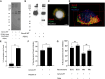
Mycobacterium tuberculosis (Mtb)-specific γ9δ2 T cells secrete granzyme A (GzmA) protective against intracellular Mtb growth. However, GzmA-enzymatic activity is unnecessary for pathogen inhibition, and the mechanisms of GzmA-mediated protection remain unknown. We show that GzmA homodimerization is essential for opsonization of mycobacteria, altered uptake into human monocytes, and subsequent pathogen clearance within the phagolysosome. Although monomeric and homodimeric GzmA bind mycobacteria, only homodimers also bind cluster of differentiation 14 (CD14) and Toll-like receptor 4 (TLR4). Without access to surface-expressed CD14 and TLR4, GzmA fails to inhibit intracellular Mtb. Upregulation of Rab11FIP1 was associated with inhibitory activity. Furthermore, GzmA colocalized with and was regulated by protein disulfide isomerase AI (PDIA1), which cleaves GzmA homodimers into monomers and prevents Mtb inhibitory activity. These studies identify a previously unrecognized role for homodimeric GzmA structure in opsonization, phagocytosis, and elimination of Mtb in human monocytes, and they highlight PDIA1 as a potential host-directed therapy for prevention and treatment of tuberculosis, a major human disease.
Keywords: CD14; PDIA1; TLR4; granzyme A; mycobacteria.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. All authors declare that they have no conflict of interest.
Figures



References
-
- World Health Organization . Global Tuberculosis Report 2021. Available at: https://www.who.int/publications/i/item/9789240037021. Accessed 18 August 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

