Radiation induces long-term muscle fibrosis and promotes a fibrotic phenotype in fibro-adipogenic progenitors
- PMID: 37671686
- PMCID: PMC10570115
- DOI: 10.1002/jcsm.13320
Radiation induces long-term muscle fibrosis and promotes a fibrotic phenotype in fibro-adipogenic progenitors
Abstract
Background: Radiation-induced muscle pathology, characterized by muscle atrophy and fibrotic tissue accumulation, is the most common debilitating late effect of therapeutic radiation exposure particularly in juvenile cancer survivors. In healthy muscle, fibro/adipogenic progenitors (FAPs) are required for muscle maintenance and regeneration, while in muscle pathology FAPs are precursors for exacerbated extracellular matrix deposition. However, the role of FAPs in radiation-induced muscle pathology has not previously been explored.
Methods: Four-week-old Male CBA or C57Bl/6J mice received a single dose (16 Gy) of irradiation (IR) to a single hindlimb with the shielded contralateral limb (CLTR) serving as a non-IR control. Mice were sacrificed 3, 7, 14 (acute IR response), and 56 days post-IR (long-term IR response). Changes in skeletal muscle morphology, myofibre composition, muscle niche cellular dynamics, DNA damage, proliferation, mitochondrial respiration, and metabolism and changes in progenitor cell fate where assessed.
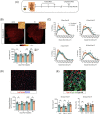
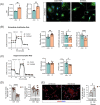
Results: Juvenile radiation exposure resulted in smaller myofibre cross-sectional area, particularly in type I and IIA myofibres (P < 0.05) and reduced the proportion of type I myofibres (P < 0.05). Skeletal muscle fibrosis (P < 0.05) was evident at 56 days post-IR. The IR-limb had fewer endothelial cells (P < 0.05) and fibro-adipogenic progenitors (FAPs) (P < 0.05) at 56 days post-IR. Fewer muscle satellite (stem) cells were detected at 3 and 56 days in the IR-limb (P < 0.05). IR induced FAP senescence (P < 0.05), increased their fibrogenic differentiation (P < 0.01), and promoted their glycolytic metabolism. Further, IR altered the FAP secretome in a manner that impaired muscle satellite (stem) cell differentiation (P < 0.05) and fusion (P < 0.05).
Conclusions: Our study suggests that following juvenile radiation exposure, FAPs contribute to long-term skeletal muscle atrophy and fibrosis. These findings provide rationale for investigating FAP-targeted therapies to ameliorate the negative late effects of radiation exposure in skeletal muscle.
Keywords: Atrophy; Differentiation; Extracellular matrix; Mesenchymal progenitors; Metabolism; Myofibroblast; Skeletal muscle.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Canadian Cancer Society's Advisory Committee on Cancer Statistics . Canadian Cancer Statistics (2015).
-
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363–385. - PubMed
-
- Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol 1985;1992:2538–2543. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

