The immunogenicity of the ChAdOx1 nCoV-19 vaccination in participants with underlying comorbidity diseases: A prospective cohort study
- PMID: 37671943
- PMCID: PMC10484043
- DOI: 10.1080/21645515.2023.2251850
The immunogenicity of the ChAdOx1 nCoV-19 vaccination in participants with underlying comorbidity diseases: A prospective cohort study
Abstract
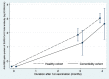
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increases mortality rates in older adults and those with comorbidities. Individuals with certain comorbidities may have a poor immune response and require early booster vaccines. We aimed to assess the immune response after two doses of ChAdOx1 nCoV-19 vaccine, at 84-day intervals, in participants with the following comorbidities; diabetes mellitus; obesity; cardiovascular disease; chronic kidney disease; rheumatological disease; cirrhosis; hematological disease; hematological malignancy; or solid malignancy. The study was conducted at Chulabhorn Hospital in Thailand, with healthy healthcare workers serving as the control group. Of the 769 participants, 352 were in the healthy cohort and 417 were in the comorbidity cohort, all received at least one dose of vaccine. Anti-RBD total antibody levels were evaluated on Day 0, Day 84, and Day 112. The results at Day 112 (4 weeks after the second dose) showed that individuals with comorbidities had a poor immune response compared to healthy individuals, especially those with hematological malignancy and solid malignancy. The geometric mean concentration (GMC) of anti-RBD antibody in the comorbidity cohort was significantly lower than that in the healthy cohort: 433.66 BAU/ml (95% CI 334.62-562.01) versus 1096.14 BAU/ml (95% CI 1010.26-1189.33), respectively. The geometric mean ratio (GMR) between the two cohorts was 0.40 (95% CI 0.30-0.52, p < .001). This study concluded that individuals with comorbidities, particularly hematological and solid malignancies, had poor immune responses and may require an early booster vaccine to prevent infection and death.
Keywords: COVID-19; ChAdOx1 nCoV-19; comorbidity; immunogenicity; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- WHO . Coronavirus disease (COVID-19). 2022. https://www.who.int/health-topics/coronavirus#tab=tab_1.
-
- Pagano L, Salmanton-Garcia J, Marchesi F, Busca A, Corradini P, Hoenigl M, Klimko N, Koehler P, Pagliuca A, Passamonti F, et al. COVID-19 infection in adult patients with hematological malignancies: a European hematology association survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. doi:10.1186/s13045-021-01177-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
