Vitamin C and D supplementation in acute myeloid leukemia
- PMID: 37671973
- PMCID: PMC10685150
- DOI: 10.1182/bloodadvances.2023010559
Vitamin C and D supplementation in acute myeloid leukemia
Abstract
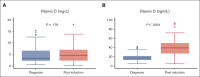
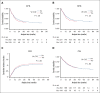
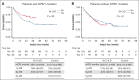
Recent studies have highlighted the role of vitamin C and D in acute myeloid leukemia (AML). In 2018, we changed our practices to add both vitamins to the supportive care for all consecutive patients with AML undergoing intensive chemotherapy. In this study, we compared the outcomes of patients treated before and after this change in practice. From 2015 to 2020, 431 patients were included, 262 of whom received no supplementation and 169 of whom received vitamin supplementation. Vitamin C and vitamin D was administered from day 10 of chemotherapy until hematologic recovery from induction and consolidation. Most patients presented at diagnosis with low levels of vitamin C and D. Upon recovery from induction, vitamin D levels among the vitamin C/D group significantly increased compared with those at diagnosis, and pretransplant levels were significantly higher in the vitamin C/D group compared with the control group (median of 33 vs 19 ng/mL; P < .0001). During induction, the rates of bacterial or fungal infection, hemorrhage, or macrophage activation syndrome were lower in the vitamin C/D group, whereas there was no difference in response rate, relapse incidence, and overall survival (OS). However, the multivariate analysis for OS showed a significant interaction between vitamin C/D and NPM1 mutation, meaning that vitamin C/D supplementation was significantly and independently associated with better OS in patients with NPM1 mutations (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.30-0.90; P = .019) compared with patients with wild-type NPM1 (HR, 1.01; 95% CI, 0.68-1.51; P = .95). In conclusion, vitamin C/D supplementation is safe and could influence the outcomes of patients with AML undergoing intensive chemotherapy.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.R. reports receiving research grants (to institution) from AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, IQVIA; and serves on advisory boards of AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, Novartis, Servier, Takeda. S.B. serves in an advisory role for AbbVie, Jazz Pharmaceuticals, Daiichi-Sankyo, Sanofi, Astellas, and Bristol Myers Squibb. F.V. received research grants from Pierre Fabre and Roche; and serves as an adviser for Astellas and Amgen. F.H. reports consultancy for Novartis, Pfizer, and Incyte; and received honoraria from Amgen and Servier. I.L. serves in an advisory role for Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures
References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

