Methodological and ethical challenges in the use of focused ultrasound for blood-brain barrier disruption in neuro-oncology
- PMID: 37672093
- PMCID: PMC10739192
- DOI: 10.1007/s00701-023-05782-5
Methodological and ethical challenges in the use of focused ultrasound for blood-brain barrier disruption in neuro-oncology
Abstract
Background: Focused ultrasound (FUS) shows promise for enhancing drug delivery to the brain by temporarily opening the blood-brain barrier (BBB), and it is increasingly used in the clinical setting to treat brain tumours. It remains however unclear whether FUS is being introduced in an ethically and methodologically sound manner. The IDEAL-D framework for the introduction of surgical innovations and the SYRCLE and ROBINS-I tools for assessing the risk of bias in animal studies and non-randomized trials, respectively, provide a comprehensive evaluation for this.
Objectives and methods: A comprehensive literature review on FUS in neuro-oncology was conducted. Subsequently, the included studies were evaluated using the IDEAL-D framework, SYRCLE, and ROBINS-I tools.
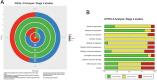
Results: In total, 19 published studies and 12 registered trials were identified. FUS demonstrated successful BBB disruption, increased drug delivery, and improved survival rates. However, the SYRCLE analysis revealed a high risk of bias in animal studies, while the ROBINS-I analysis found that most human studies had a high risk of bias due to a lack of blinding and heterogeneous samples. Of the 15 pre-clinical stage 0 studies, only six had formal ethical approval, and only five followed animal care policies. Both stage 1 studies and stage 1/2a studies failed to provide information on patient data confidentiality. Overall, no animal or human study reached the IDEAL-D stage endpoint.
Conclusion: FUS holds promise for enhancing drug delivery to the brain, but its development and implementation must adhere to rigorous safety standards using the established ethical and methodological frameworks. The complementary use of IDEAL-D, SYRCLE, and ROBINS-I tools indicates a high risk of bias and ethical limitations in both animal and human studies, highlighting the need for further improvements in study design for a safe implementation of FUS in neuro-oncology.
Keywords: Brain tumor; Focused ultrasound; Glioblastoma; Glioma; IDEAL; Neuro-oncology; ROBINS-I; SYRCLE.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Carpentier A, Canney M, Vignot A et al (2016) Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8(343). 10.1126/SCITRANSLMED.AAF6086/SUPPL_FILE/8-343RE2_SM.PDF - PubMed
-
- Conti A, Kamimura HAS, Novell A, Duggento A, Toschi N (2020) Magnetic resonance methods for focused ultrasound-induced blood-brain barrier opening. Front Phys 8. 10.3389/fphy.2020.547674
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

