Neuronal maturation-dependent nano-neuro interaction and modulation
- PMID: 37672212
- PMCID: PMC10615777
- DOI: 10.1039/d3nh00258f
Neuronal maturation-dependent nano-neuro interaction and modulation
Abstract
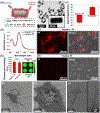
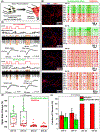
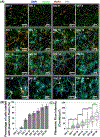
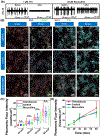
Nanotechnology-enabled neuromodulation is a promising minimally-invasive tool in neuroscience and engineering for both fundamental studies and clinical applications. However, the nano-neuro interaction at different stages of maturation of a neural network and its implications for the nano-neuromodulation remain unclear. Here, we report heterogeneous to homogeneous transformation of neuromodulation in a progressively maturing neural network. Utilizing plasmonic-fluors as ultrabright fluorescent nanolabels, we reveal that negative surface charge of nanoparticles renders selective nano-neuro interaction with a strong correlation between the maturation stage of the individual neurons in the neural network and the density of the nanoparticles bound on the neurons. In stark contrast to homogeneous neuromodulation in a mature neural network reported so far, the maturation-dependent density of the nanoparticles bound to neurons in a developing neural network resulted in a heterogeneous optical neuromodulation (i.e., simultaneous excitation and inhibition of neural network activity). This study advances our understanding of nano-neuro interactions and nano-neuromodulation with potential applications in minimally-invasive technologies for treating neuronal disorders in parts of the mammalian brain where neurogenesis persists throughout aging.
Conflict of interest statement
Competing Interests
The authors declare the following competing financial interests: S.S. is one of the inventors on a pending patent related to plasmonic fluor technology and the technology has been licensed by the Office of Technology Management at Washington University in St Louis to Auragent Bioscience LLC, which is developing plasmonic fluor products. S.S. is one of the co-founders and shareholders of Auragent Bioscience LLC. These potential conflicts of interest have been disclosed and are being managed by Washington University in St Louis.
Figures



Similar articles
-
Reversible Photothermal Modulation of Electrical Activity of Excitable Cells using Polydopamine Nanoparticles.Adv Mater. 2021 Aug;33(32):e2008809. doi: 10.1002/adma.202008809. Epub 2021 Jul 3. Adv Mater. 2021. PMID: 34216406 Free PMC article.
-
Neuro-nanotechnology: diagnostic and therapeutic nano-based strategies in applied neuroscience.Biomed Eng Online. 2023 Jan 2;22(1):1. doi: 10.1186/s12938-022-01062-y. Biomed Eng Online. 2023. PMID: 36593487 Free PMC article. Review.
-
Nanotechnology Facilitated Cultured Neuronal Network and Its Applications.Int J Mol Sci. 2021 May 24;22(11):5552. doi: 10.3390/ijms22115552. Int J Mol Sci. 2021. PMID: 34074027 Free PMC article. Review.
-
Exploring Present and Future Directions in Nano-Enhanced Optoelectronic Neuromodulation.Acc Chem Res. 2024 May 7;57(9):1398-1410. doi: 10.1021/acs.accounts.4c00086. Epub 2024 Apr 23. Acc Chem Res. 2024. PMID: 38652467 Free PMC article. Review.
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
Cited by
-
Size-Optimized Layered Double Hydroxide Nanoparticles Promote Neural Progenitor Cells Differentiation of Embryonic Stem Cells Through the Regulation of M6A Methylation.Int J Nanomedicine. 2024 May 13;19:4181-4197. doi: 10.2147/IJN.S463141. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38766656 Free PMC article.
-
Plasmonic nanoparticle sensors: current progress, challenges, and future prospects.Nanoscale Horiz. 2024 Nov 19;9(12):2085-2166. doi: 10.1039/d4nh00226a. Nanoscale Horiz. 2024. PMID: 39240539 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

