Hyperthermic Intraperitoneal Chemotherapy After Interval Cytoreductive Surgery for Patients With Advanced-Stage Ovarian Cancer Who Had Received Neoadjuvant Chemotherapy
- PMID: 37672264
- PMCID: PMC10483378
- DOI: 10.1001/jamasurg.2023.3944
Hyperthermic Intraperitoneal Chemotherapy After Interval Cytoreductive Surgery for Patients With Advanced-Stage Ovarian Cancer Who Had Received Neoadjuvant Chemotherapy
Abstract
Importance: Hyperthermic intraperitoneal chemotherapy (HIPEC) followed by interval cytoreductive surgery (ICS) has shown survival benefits for patients with advanced-stage ovarian cancer. However, there is still a lack of consensus regarding the integration of HIPEC into clinical practice.
Objective: To evaluate the safety and effectiveness of ICS with HIPEC compared with ICS alone in clinical practice for patients with advanced-stage ovarian cancer.
Design, setting, and participants: This prospective, multicenter, comparative effectiveness cohort study enrolled 205 patients with stage III or IV ovarian cancer who had received at least 3 cycles of neoadjuvant chemotherapy followed by ICS with HIPEC or ICS without HIPEC at 7 Korean Gynecologic Oncology Group institutions between September 1, 2017, and April 22, 2022. Nine patients were excluded because they did not meet the inclusion criteria.
Exposures: Neoadjuvant chemotherapy followed by ICS with HIPEC or ICS without HIPEC.
Main outcomes and measures: The primary end point was progression-free survival (PFS). Overall survival (OS) and the safety profile were the key secondary end points.
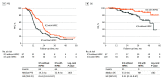
Results: This study included 196 patients (median age, 58.0 years [range, 38-82 years]), of whom 109 underwent ICS with HIPEC and 87 underwent ICS without HIPEC. The median duration of follow-up was 28.2 months (range, 3.5-58.6 months). Disease recurrence occurred in 128 patients (65.3%), and 30 patients (15.3%) died. Interval cytoreductive surgery with HIPEC was associated with a significant improvement in median PFS compared with ICS without HIPEC (22.9 months [95% CI, 3.5-58.6 months] vs 14.2 months [95% CI, 4.0-56.2 months]; P = .005) and median OS (not reached [95% CI, 3.5 months to not reached] vs 53.0 [95% CI, 4.6-56.2 months]; P = .002). The frequency of grade 3 or 4 postoperative complications was similar in both groups (ICS with HIPEC, 3 of 109 [2.8%] vs ICS without HIPEC, 3 of 87 [3.4%]; P > .99). Among patients with recurrence, the frequency of peritoneal recurrence was lower in the ICS with HIPEC group than in the ICS without HIPEC group (21 of 64 [32.8%] vs 41 of 64 [64.1%]; P = .001).
Conclusions and relevance: This study suggests that ICS in conjunction with HIPEC was associated with longer PFS and OS than ICS without HIPEC for patients with advanced-stage ovarian cancer and was not associated with higher rates of postoperative complications. The lower rate of peritoneal recurrence after HIPEC may be associated with improved OS.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

