Lignin conversion to β-ketoadipic acid by Pseudomonas putida via metabolic engineering and bioprocess development
- PMID: 37672573
- PMCID: PMC10482344
- DOI: 10.1126/sciadv.adj0053
Lignin conversion to β-ketoadipic acid by Pseudomonas putida via metabolic engineering and bioprocess development
Abstract
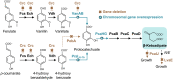
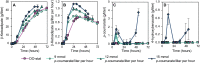
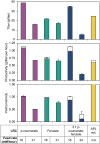
Bioconversion of a heterogeneous mixture of lignin-related aromatic compounds (LRCs) to a single product via microbial biocatalysts is a promising approach to valorize lignin. Here, Pseudomonas putida KT2440 was engineered to convert mixed p-coumaroyl- and coniferyl-type LRCs to β-ketoadipic acid, a precursor for performance-advantaged polymers. Expression of enzymes mediating aromatic O-demethylation, hydroxylation, and ring-opening steps was tuned, and a global regulator was deleted. β-ketoadipate titers of 44.5 and 25 grams per liter and productivities of 1.15 and 0.66 grams per liter per hour were achieved from model LRCs and corn stover-derived LRCs, respectively, the latter representing an overall yield of 0.10 grams per gram corn stover-derived lignin. Technoeconomic analysis of the bioprocess and downstream processing predicted a β-ketoadipate minimum selling price of $2.01 per kilogram, which is cost competitive with fossil carbon-derived adipic acid ($1.10 to 1.80 per kilogram). Overall, this work achieved bioproduction metrics with economic relevance for conversion of lignin-derived streams into a performance-advantaged bioproduct.
Figures





References
-
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan, C. E. Wyman, Lignin valorization: Improving lignin processing in the biorefinery. Science 344, 1246843 (2014). - PubMed
-
- A. Corona, M. J. Biddy, D. R. Vardon, M. Birkved, M. Z. Hauschild, G. T. Beckham, Life cycle assessment of adipic acid production from lignin. Green Chem. 20, 3857–3866 (2018).
-
- R. Davis, A. Bartling, Biochemical conversion of lignocellulosic biomass to hydrocarbon fuels and products: 2021 state of technology and future research (National Renewable Energy Laboratory, 2022).
-
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius, B. M. Weckhuysen, The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

