Nucleoside diphosphate kinases 1 and 2 regulate a protective liver response to a high-fat diet
- PMID: 37672589
- PMCID: PMC10482350
- DOI: 10.1126/sciadv.adh0140
Nucleoside diphosphate kinases 1 and 2 regulate a protective liver response to a high-fat diet
Abstract
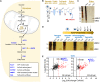
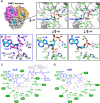
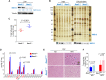
The synthesis of fatty acids from acetyl-coenzyme A (AcCoA) is deregulated in diverse pathologies, including cancer. Here, we report that fatty acid accumulation is negatively regulated by nucleoside diphosphate kinases 1 and 2 (NME1/2), housekeeping enzymes involved in nucleotide homeostasis that were recently found to bind CoA. We show that NME1 additionally binds AcCoA and that ligand recognition involves a unique binding mode dependent on the CoA/AcCoA 3' phosphate. We report that Nme2 knockout mice fed a high-fat diet (HFD) exhibit excessive triglyceride synthesis and liver steatosis. In liver cells, NME2 mediates a gene transcriptional response to HFD leading to the repression of fatty acid accumulation and activation of a protective gene expression program via targeted histone acetylation. Our findings implicate NME1/2 in the epigenetic regulation of a protective liver response to HFD and suggest a potential role in controlling AcCoA usage between the competing paths of histone acetylation and fatty acid synthesis.
Figures




References
-
- M. Wallace, C. M. Metallo, Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin. Cell Dev. Biol. 108, 65–71 (2020). - PubMed
-
- F. Ameer, L. Scandiuzzi, S. Hasnain, H. Kalbacher, N. Zaidi, De novo lipogenesis in health and disease. Metabolism 63, 895–902 (2014). - PubMed
-
- G. I. Smith, M. Shankaran, M. Yoshino, G. G. Schweitzer, M. Chondronikola, J. W. Beals, A. L. Okunade, B. W. Patterson, E. Nyangau, T. Field, C. B. Sirlin, S. Talukdar, M. K. Hellerstein, S. Klein, Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 130, 1453–1460 (2020). - PMC - PubMed
-
- K. H. M. Roumans, L. Lindeboom, P. Veeraiah, C. M. E. Remie, E. Phielix, B. Havekes, Y. M. H. Bruls, M. Brouwers, M. Stahlman, M. Alssema, H. P. F. Peters, R. de Mutsert, B. Staels, M. R. Taskinen, J. Boren, P. Schrauwen, V. B. Schrauwen-Hinderling, Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat. Commun. 11, 1891 (2020). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

