The Sympathetic Nervous System Promotes Hepatic Lymphangiogenesis, which Is Protective Against Liver Fibrosis
- PMID: 37673329
- PMCID: PMC10699132
- DOI: 10.1016/j.ajpath.2023.08.004
The Sympathetic Nervous System Promotes Hepatic Lymphangiogenesis, which Is Protective Against Liver Fibrosis
Abstract
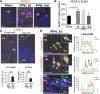
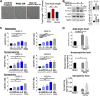
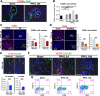
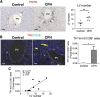
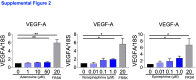
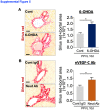
Liver is the largest lymph-producing organ. In cirrhotic patients, lymph production significantly increases concomitant with lymphangiogenesis. The aim of this study was to determine the mechanism of lymphangiogenesis in liver and its implication in liver fibrosis. Liver biopsies from portal hypertensive patients with portal-sinusoidal vascular disease (n = 22) and liver cirrhosis (n = 5) were evaluated for lymphangiogenesis and compared with controls (n = 9 and n = 6, respectively). For mechanistic studies, rats with partial portal vein ligation (PPVL) and bile duct ligation (BDL) were used. A gene profile data set (GSE77627), including 14 histologically normal liver, 18 idiopathic noncirrhotic portal hypertension, and 22 cirrhotic patients, was analyzed. Lymphangiogenesis was significantly increased in livers from patients with portal-sinusoidal vascular disease, cirrhotic patients, as well as PPVL and BDL rats. Importantly, Schwann cells of sympathetic nerves highly expressed vascular endothelial growth factor-C in PPVL rats. Vascular endothelial growth factor-C neutralizing antibody or sympathetic denervation significantly decreased lymphangiogenesis in livers of PPVL and BDL rats, which resulted in progression of liver fibrosis. Liver specimens from cirrhotic patients showed a positive correlation between sympathetic nerve/Schwann cell-positive areas and lymphatic vessel numbers, which was supported by gene set analysis from patients with noncirrhotic portal hypertension and cirrhotic patients. Sympathetic nerves promote hepatic lymphangiogenesis in noncirrhotic and cirrhotic livers. Increased hepatic lymphangiogenesis can be protective against liver fibrosis.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures









References
-
- Alitalo K., Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. - PubMed
-
- Tammela T., Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. - PubMed
-
- Ritchie H.D., Grindlay J.H., Bollman J.L. Flow of lymph from the canine liver. Am J Physiol. 1959;196:105–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

