Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study
- PMID: 37673431
- PMCID: PMC10480689
- DOI: 10.1136/bmj-2022-074450
Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: nationwide cohort study
Abstract
Objective: To study the influence of concomitant use of hormonal contraception and non-steroidal anti-inflammatory drugs (NSAIDs) on the risk of venous thromboembolism.
Design: Nationwide cohort study.
Setting: Denmark through national registries.
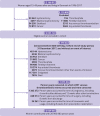
Participants: All 15-49 year old women living in Denmark between 1996 and 2017 with no medical history of any venous or arterial thrombotic event, cancer, thrombophilia, hysterectomy, bilateral oophorectomy, sterilisation, or infertility treatment (n=2 029 065).
Main outcome measure: A first time discharge diagnosis of lower limb deep venous thrombosis or pulmonary embolism.
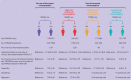
Results: Among 2.0 million women followed for 21.0 million person years, 8710 venous thromboembolic events occurred. Compared with non-use of NSAIDs, use of NSAIDs was associated with an adjusted incidence rate ratio of venous thromboembolism of 7.2 (95% confidence interval 6.0 to 8.5) in women not using hormonal contraception, 11.0 (9.6 to 12.6) in women using high risk hormonal contraception, 7.9 (5.9 to 10.6) in those using medium risk hormonal contraception, and 4.5 (2.6 to 8.1) in users of low/no risk hormonal contraception. The corresponding numbers of extra venous thromboembolic events per 100 000 women over the first week of NSAID treatment compared with non-use of NSAIDs were 4 (3 to 5) in women not using hormonal contraception, 23 (19 to 27) in women using high risk hormonal contraception, 11 (7 to 15) in those using medium risk hormonal contraception, and 3 (0 to 5) in users of low/no risk hormonal contraception.
Conclusions: NSAID use was positively associated with the development of venous thromboembolism in women of reproductive age. The number of extra venous thromboembolic events with NSAID use compared with non-use was significantly larger with concomitant use of high/medium risk hormonal contraception compared with concomitant use of low/no risk hormonal contraception. Women needing both hormonal contraception and regular use of NSAIDs should be advised accordingly.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Danish Heart Foundation, which funded AM’s salary, including this study; LSM has received grants from the health insurance organisation “Denmark,” the Danish Cancer Society’s Scientific Committee, and Novo Nordisk for research unrelated to the present study; CTP has received grants from Novo Nordisk and Bayer outside the current study; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
NSAIDs, hormonal contraception, and venous thromboembolism.BMJ. 2023 Sep 6;382:1990. doi: 10.1136/bmj.p1990. BMJ. 2023. PMID: 37673432 No abstract available.
-
NSAIDs and hormonal contraceptives are linked to VTE in women with no previous thrombotic disease.Ann Intern Med. 2023 Dec;176(12):JC143. doi: 10.7326/J23-0099. Epub 2023 Dec 5. Ann Intern Med. 2023. PMID: 38048580
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
