A super-enhancer-regulated RNA-binding protein cascade drives pancreatic cancer
- PMID: 37673892
- PMCID: PMC10482938
- DOI: 10.1038/s41467-023-40798-6
A super-enhancer-regulated RNA-binding protein cascade drives pancreatic cancer
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy in need of new therapeutic options. Using unbiased analyses of super-enhancers (SEs) as sentinels of core genes involved in cell-specific function, here we uncover a druggable SE-mediated RNA-binding protein (RBP) cascade that supports PDAC growth through enhanced mRNA translation. This cascade is driven by a SE associated with the RBP heterogeneous nuclear ribonucleoprotein F, which stabilizes protein arginine methyltransferase 1 (PRMT1) to, in turn, control the translational mediator ubiquitin-associated protein 2-like. All three of these genes and the regulatory SE are essential for PDAC growth and coordinately regulated by the Myc oncogene. In line with this, modulation of the RBP network by PRMT1 inhibition reveals a unique vulnerability in Myc-high PDAC patient organoids and markedly reduces tumor growth in male mice. Our study highlights a functional link between epigenetic regulation and mRNA translation and identifies components that comprise unexpected therapeutic targets for PDAC.
© 2023. Springer Nature Limited.
Conflict of interest statement
G.W.Y. is a co-founder, member of the Board of Directors, on the scientific advisory boards, equity holder, and paid consultant for Locanabio and Eclipse BioInnovations. G.W.Y. is a visiting professor at the National University of Singapore. G.W.Y.’s interests have been reviewed and approved by the University of California, San Diego, in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Figures

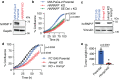
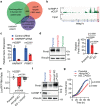
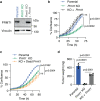




References
Publication types
MeSH terms
Substances
Grants and funding
- P42 ES010337/ES/NIEHS NIH HHS/United States
- T32 CA009370/CA/NCI NIH HHS/United States
- U41 HG009889/HG/NHGRI NIH HHS/United States
- U24 HG009889/HG/NHGRI NIH HHS/United States
- R01 HG004659/HG/NHGRI NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- R01 CA220468/CA/NCI NIH HHS/United States
- T32 CA067754/CA/NCI NIH HHS/United States
- P01 CA265762/CA/NCI NIH HHS/United States
- P01 HL088093/HL/NHLBI NIH HHS/United States
- R01 CA155620/CA/NCI NIH HHS/United States
- R01 DK057978/DK/NIDDK NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

