Cold-activated brown fat-derived extracellular vesicle-miR-378a-3p stimulates hepatic gluconeogenesis in male mice
- PMID: 37673898
- PMCID: PMC10482845
- DOI: 10.1038/s41467-023-41160-6
Cold-activated brown fat-derived extracellular vesicle-miR-378a-3p stimulates hepatic gluconeogenesis in male mice
Abstract
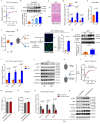
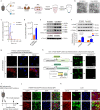
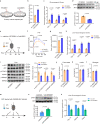
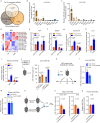
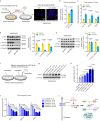
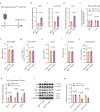
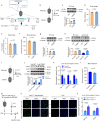
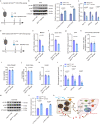
During cold exposure, activated brown adipose tissue (BAT) takes up a large amount of circulating glucose to fuel non-shivering thermogenesis and defend against hypothermia. However, little is known about the endocrine function of BAT controlling glucose homoeostasis under this thermoregulatory challenge. Here, we show that in male mice, activated BAT-derived extracellular vesicles (BDEVs) reprogram systemic glucose metabolism by promoting hepatic gluconeogenesis during cold stress. Cold exposure facilitates the selective packaging of miR-378a-3p-one of the BAT-enriched miRNAs-into EVs and delivery into the liver. BAT-derived miR-378a-3p enhances gluconeogenesis by targeting p110α. miR-378 KO mice display reduced hepatic gluconeogenesis during cold exposure, while restoration of miR-378a-3p in iBAT induces the expression of gluconeogenic genes in the liver. These findings provide a mechanistic understanding of BDEV-miRNA as stress-induced batokine to coordinate systemic glucose homoeostasis. This miR-378a-3p-mediated interorgan communication highlights a novel endocrine function of BAT in preventing hypoglycemia during cold stress.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

