Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells
- PMID: 37674084
- PMCID: PMC10533412
- DOI: 10.1038/s41586-023-06525-3
Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells
Abstract
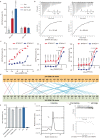
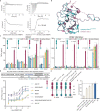
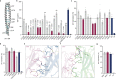
In both cancer and infections, diseased cells are presented to human Vγ9Vδ2 T cells through an 'inside out' signalling process whereby structurally diverse phosphoantigen (pAg) molecules are sensed by the intracellular domain of butyrophilin BTN3A11-4. Here we show how-in both humans and alpaca-multiple pAgs function as 'molecular glues' to promote heteromeric association between the intracellular domains of BTN3A1 and the structurally similar butyrophilin BTN2A1. X-ray crystallography studies visualized that engagement of BTN3A1 with pAgs forms a composite interface for direct binding to BTN2A1, with various pAg molecules each positioned at the centre of the interface and gluing the butyrophilins with distinct affinities. Our structural insights guided mutagenesis experiments that led to disruption of the intracellular BTN3A1-BTN2A1 association, abolishing pAg-mediated Vγ9Vδ2 T cell activation. Analyses using structure-based molecular-dynamics simulations, 19F-NMR investigations, chimeric receptor engineering and direct measurement of intercellular binding force revealed how pAg-mediated BTN2A1 association drives BTN3A1 intracellular fluctuations outwards in a thermodynamically favourable manner, thereby enabling BTN3A1 to push off from the BTN2A1 ectodomain to initiate T cell receptor-mediated γδ T cell activation. Practically, we harnessed the molecular-glue model for immunotherapeutics design, demonstrating chemical principles for developing both small-molecule activators and inhibitors of human γδ T cell function.
© 2023. The Author(s).
Conflict of interest statement
Y.Z. and W.M. are co-founders of Unicet Biotech, which is engaged in γδ T cell immunotherapy development.
Figures










References
-
- Yang Y, et al. A structural change in butyrophilin upon phosphoantigen binding underlies phosphoantigen-mediated Vγ9Vδ2 T cell activation. Immunity. 2019;50:1043–1053. - PubMed
-
- Rossjohn J, et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015;33:169–200. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

