Multicenter, randomized controlled, open label evaluation of the efficacy and safety of arbidol hydrochloride tablets in the treatment of influenza-like cases
- PMID: 37674112
- PMCID: PMC10483848
- DOI: 10.1186/s12879-023-08570-9
Multicenter, randomized controlled, open label evaluation of the efficacy and safety of arbidol hydrochloride tablets in the treatment of influenza-like cases
Abstract
Objective: To study the efficacy and safety of arbidol hydrochloride tablets as a treatment for influenza-like diseases.
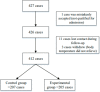
Methods: In this multicenter, randomized, controlled, open label study, a total of 412 influenza-like cases were collected from 14 hospitals in seven regions of Hebei Province from September 2021 to March 2022. Patients were randomly divided into two groups. The control group (n = 207) were administered oseltamivir phosphate capsules for five days and the experimental group (n = 205) were administered arbidol hydrochloride tablets for five days. The primary endpoint was the time to normal body temperature, and the secondary endpoints included the time to remission of influenza symptoms, incidence of influenza-like complications, and incidence of adverse reactions.
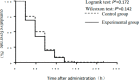
Results: Before treatment, there was no significant difference between the two groups in general conditions, blood routine, body temperature, or symptom severity. After treatment, there was no significant difference between the groups in the mean time to fever remission (59.24 h ± 25.21 vs. 61.05 h ± 29.47) or the mean time to remission of influenza symptoms (57.31 h ± 30.19 vs. 62.02 h ± 32.08). Survival analyses using Log-rank and Wilcoxon bilateral tests showed that there was no significant difference in fever relief time or influenza symptom relief time between the two groups. Regarding the incidence of complications and adverse events, there was only one case of tracheitis, one case of nausea, one case of vomiting, and one case of dizziness in the control group. In the experimental group, there was one case of nausea, one case of vomiting, and one case of drowsiness. In addition, one patient in the control group was hospitalized for urinary calculi.
Conclusion: There was no significant difference between the patients with influenza-like cases treated with arbidol hydrochloride tablets and those treated with oseltamivir phosphate capsules. Further, the patients treated with arbidol hydrochloride tablets had fewer adverse reactions, and thus, the tablets were safe to use.
Keywords: Arbidol hydrochloride tablets; Influenza-like cases; Multicenter study; Randomized controlled trial.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- National Immunization Program Technical Working Group influenza vaccine working group Technical guidelines for influenza vaccination in China (2019–2020) Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40(11):1333–49. - PubMed
-
- World Health Organization. Fact sheet on influenza(seasonal)[EB/OL]. 2020, https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).
-
- World Health Organization. WHO Model List of Essential Medicines. 2021, https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02. Accessed 30 Sept 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical