Green SPIONs as a novel highly selective treatment for leishmaniasis: an in vitro study against Leishmania amazonensis intracellular amastigotes
- PMID: 37674544
- PMCID: PMC10477971
- DOI: 10.3762/bjnano.14.73
Green SPIONs as a novel highly selective treatment for leishmaniasis: an in vitro study against Leishmania amazonensis intracellular amastigotes
Abstract
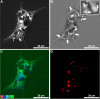
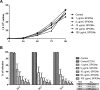
The main goal of this work was to evaluate the therapeutic potential of green superparamagnetic iron oxide nanoparticles (SPIONs) produced with coconut water for treating cutaneous leishmaniasis caused by Leishmania amazonensis. Optical and electron microscopy techniques were used to evaluate the effects on cell proliferation, infectivity percentage, and ultrastructure. SPIONs were internalized by both parasite stages, randomly distributed in the cytosol and located mainly in membrane-bound compartments. The selectivity index for intracellular amastigotes was more than 240 times higher compared to current drugs used to treat the disease. The synthesized SPIONs showed promising activity against Leishmania and can be considered a strong candidate for a new therapeutic approach for treating leishmaniases.
Keywords: Leishmania amazonensis; Leishmaniasis; SPIONs; coconut water; nanomedicine.
Copyright © 2023, Verçoza et al.
Figures



References
-
- World Health Organization (WHO) Disease factsheet 2019 – Leishmaniasis. 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
-
- Ponte-Sucre A, Padrón-Nieves M, editors. Drug Resistance in Leishmania Parasites. Cham, Switzerland: Springer International Publishing; 2018. - DOI
LinkOut - more resources
Full Text Sources
