Multisystemic RFC1-Related Disorder: Expanding the Phenotype Beyond Cerebellar Ataxia, Neuropathy, and Vestibular Areflexia Syndrome
- PMID: 37674869
- PMCID: PMC10479936
- DOI: 10.1212/CPJ.0000000000200190
Multisystemic RFC1-Related Disorder: Expanding the Phenotype Beyond Cerebellar Ataxia, Neuropathy, and Vestibular Areflexia Syndrome
Abstract
Background and objectives: The RFC1 spectrum has become considerably expanded as multisystemic features beyond the triad of cerebellar ataxia, neuropathy, and vestibular areflexia syndrome (CANVAS) have started to be unveiled, although many still require clinical replication. Here, we aimed to clinically characterize a cohort of RFC1-positive patients by addressing both classic and multisystemic features. In a second part of this study, we prospectively assessed small nerve fibers (SNF) and autonomic function in a subset of these RFC1-related patients.
Methods: We retrospectively enrolled 67 RFC1-positive patients from multiple neurologic centers in Portugal. All patients underwent full neurologic and vestibular evaluation, as well as neuroimaging and neurophysiologic studies. For SNF and autonomic testing (n = 15), we performed skin biopsies, quantitative sensory testing, sudoscan, sympathetic skin response, heart rate deep breathing, and tilt test.
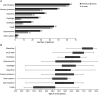
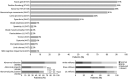
Results: Multisystemic features beyond CANVAS were present in 82% of the patients, mainly chronic cough (66%) and dysautonomia (43%). Other features included motor neuron (MN) affection and motor neuropathy (18%), hyperkinetic movement disorders (16%), sleep apnea (6%), REM and non-REM sleep disorders (5%), and cranial neuropathy (5%). Ten patients reported an inverse association between cough and ataxia severity. A very severe epidermal denervation was found in skin biopsies of all patients. Autonomic dysfunction comprised cardiovascular (67%), cardiovagal (54%), and/or sudomotor (50%) systems.
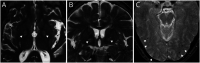
Discussion: The presence of MN involvement, motor neuropathy, small fiber neuropathy, or extrapyramidal signs should not preclude RFC1 testing in cases of sensory neuronopathy. Indeed, the RFC1 spectrum can overlap not only with multiple system atrophy but also with hereditary motor and sensory neuropathy, hereditary sensory and autonomic neuropathy, and feeding dystonia phenotypes. Some clinical-paraclinical dissociations can pose diagnostic challenges, namely large and small fiber neuropathy and sudomotor dysfunction which are usually subclinical.
© 2023 American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Figures

References
LinkOut - more resources
Full Text Sources
