Parasympathetic and sympathetic axons are bundled in the cardiac ventricles and undergo physiological reinnervation during heart regeneration
- PMID: 37674983
- PMCID: PMC10477065
- DOI: 10.1016/j.isci.2023.107709
Parasympathetic and sympathetic axons are bundled in the cardiac ventricles and undergo physiological reinnervation during heart regeneration
Abstract
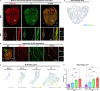
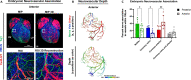
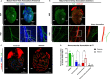
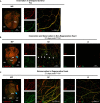
Sympathetic innervation influences homeostasis, repair, and pathology in the cardiac ventricles; in contrast, parasympathetic innervation is considered to have minimal contribution and influence in the ventricles. Here, we use genetic models, whole-mount imaging, and three-dimensional modeling to define cardiac nerve architecture during development, disease, and regeneration. Our approach reveals that parasympathetic nerves extensively innervate the cardiac ventricles. Furthermore, we identify that parasympathetic and sympathetic axons develop synchronously and are bundled throughout the ventricles. We further investigate cardiac nerve remodeling in the regenerative neonatal and the non-regenerative postnatal mouse heart. Our results show that the regenerating myocardium undergoes a unique process of physiological reinnervation, where proper nerve distribution and architecture is reestablished, in stark contrast to the non-regenerating heart. Mechanistically, we demonstrate that physiological reinnervation during regeneration is dependent on collateral artery formation. Our results reveal clinically significant insights into cardiac nerve plasticity which can identify new therapies for cardiac disease.
Keywords: Cell biology; Molecular biology; Neuroscience.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Defining Cardiac Nerve Architecture During Development, Disease, and Regeneration.bioRxiv [Preprint]. 2023 Jan 3:2022.12.31.522405. doi: 10.1101/2022.12.31.522405. bioRxiv. 2023. Update in: iScience. 2023 Aug 25;26(10):107709. doi: 10.1016/j.isci.2023.107709. PMID: 36711742 Free PMC article. Updated. Preprint.
References
-
- Randall W.C. Oxford University Press; 1984. Nervous Control of Cardiovascular Function.
-
- Randall W.C. Williams and Wilkins Company; 1965. Nervous Control of the Heart.
-
- Hanna P., Dacey M.J., Brennan J., Moss A., Robbins S., Achanta S., Biscola N.P., Swid M.A., Rajendran P.S., Mori S., et al. Innervation and Neuronal Control of the Mammalian Sinoatrial Node a Comprehensive Atlas. Circ. Res. 2021;128:1279–1296. doi: 10.1161/CIRCRESAHA.120.318458. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

