Outcome of percutaneous coronary intervention using ultrathin-strut biodegradable polymer sirolimus-eluting versus thin-strut durable polymer zotarolimus-eluting stents in patients with comorbid peripheral arterial disease: a post-hoc analysis from two randomized trials
- PMID: 37675090
- PMCID: PMC10478025
- DOI: 10.21037/cdt-22-584
Outcome of percutaneous coronary intervention using ultrathin-strut biodegradable polymer sirolimus-eluting versus thin-strut durable polymer zotarolimus-eluting stents in patients with comorbid peripheral arterial disease: a post-hoc analysis from two randomized trials
Abstract
Background: In patients with peripheral arterial disease (PADs), who underwent percutaneous coronary intervention (PCI), little is known about the potential impact of using different new-generation drug-eluting stents (DES) on outcome. In PCI all-comers, the results of most between-stent comparisons-stratified by strut thickness-suggested some advantage of coronary stents with ultrathin-struts. The current post-hoc analysis aimed to assess outcomes of PCI with ultrathin-strut biodegradable polymer sirolimus-eluting stents (BP-SES) vs. thin-strut durable polymer zotarolimus-eluting stents (DP-ZES) in patients with PADs.
Methods: We pooled 3-year patient-level data from two large-scale randomized all-comer trials to compare Orsiro ultrathin-strut BP-SES vs. Resolute-type thin-strut DP-ZES in trial participants with concomitant PADs. BIO-RESORT (December 2012 to August 2015) and BIONYX (October 2015 to December 2016) included all-comer patients who were aged 18 years or older, capable of providing informed consent, and required a PCI. The trials had web-based randomization, with block sizes of 4 and 8, performed in a 1:1:1 or 1:1 fashion. Assessors, research staff, and patients were blinded to the type of stent used. We assessed the composite main clinical endpoint target vessel failure [TVF: cardiac death, target vessel related myocardial infarction (MI), or clinically indicated target vessel revascularization (TVR)], its components, and stent thrombosis.
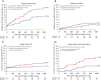
Results: Of 4,830 trial participants, 360 had PADs: 177 (49.2%) were treated with BP-SES and 183 (50.8%) with DP-ZES. Baseline characteristics were similar. For BP-SES, the 3-year TVF rate was 11.0% and for DP-ZES 17.9% [hazard ratio (HR): 0.59, 95% CI: 0.33-1.04; P=0.07]. For BP-SES, the TVR rate was lower than for DP-ZES (4.1% vs. 11.0%; HR: 0.36, 95% CI: 0.15-0.86; P=0.016), but this did not translate into between-group differences in cardiac death or MI. In small vessels (<2.75 mm), the TVR rate was also lower in BP-SES (5.6% vs. 13.9%; HR: 0.32, 95% CI: 0.11-0.91; P=0.024). Definite-or-probable stent thrombosis rates were 1.2% and 2.3% (P=0.43).
Conclusions: In PCI patients with PADs, the 3-year TVF incidence was numerically lower in the ultrathin-strut BP-SES vs. the thin-strut DP-ZES group. Furthermore, TVR risk was significantly lower in ultrathin-strut BP-SES, mainly driven by a lower TVR rate in small vessels.
Trial registration: BIO-RESORT trial: clinicaltrials.gov (NCT01674803); BIONYX trial: clinicaltrials.gov (NCT02508714).
Keywords: Coronary artery disease; drug-eluting stent; percutaneous coronary intervention (PCI); peripheral arterial disease (PADs); randomized trial.
2023 Cardiovascular Diagnosis and Therapy. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-22-584/coif). EHP reports that the Research Department of Thoraxcentrum Twente has received institutional research grants from these companies (Biotronik, Boston Scientific, and Medtronic) for performing the 2 RCTs on which the analyses of this manuscript are based. In addition, the Research Department of Thoraxcentrum Twente has received an institutional research grant for performing a stent study, not directly related with the content of the present manuscript. RLA reports a teaching grant from Biotronik, a license from Sanovi, a speaking fee from Abiomed and support from Amgen for attending a meeting, all outside the submitted work. CvB reports that the Research Department of Thoraxcentrum Twente has received institutional research grants from these companies (Biotronik, Boston Scientific, and Medtronic) for performing the 2 RCTs on which the analyses of this manuscript are based. In addition, the Research Department of Thoraxcentrum Twente has received an institutional research grant for performing a stent study, not directly related with the content of the present manuscript. The author is a DSMB member in a TAVI trial and a vascular surgical trial. The other authors have no conflicts of interest to declare.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
