How can Cytokine-induced killer cells overcome CAR-T cell limits
- PMID: 37675107
- PMCID: PMC10477668
- DOI: 10.3389/fimmu.2023.1229540
How can Cytokine-induced killer cells overcome CAR-T cell limits
Abstract
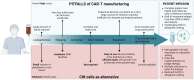
The successful treatment of patients affected by B-cell malignancies with Chimeric Antigen Receptor (CAR)-T cells represented a breakthrough in the field of adoptive cell therapy (ACT). However, CAR-T therapy is not an option for every patient, and several needs remain unmet. In particular, the production of CAR-T cells is expensive, labor-intensive and logistically challenging; additionally, the toxicities deriving from CAR-T cells infusion, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), have been documented extensively. Alternative cellular therapy products such as Cytokine-induced killer (CIK) cells have the potential to overcome some of these obstacles. CIK cells are a heterogeneous population of polyclonal CD3+CD56+ T cells with phenotypic and functional properties of NK cells. CIK cell cytotoxicity is exerted in a major histocompatibility complex (MHC)-unrestricted manner through the engagement of natural killer group 2 member D (NKG2D) molecules, against a wide range of hematological and solid tumors without the need for prior antigen exposure or priming. The foremost potential of CIK cells lies in the very limited ability to induce graft-versus-host disease (GvHD) reactions in the allogeneic setting. CIK cells are produced with a simple and extremely efficient expansion protocol, which leads to a massive expansion of effector cells and requires a lower financial commitment compared to CAR-T cells. Indeed, CAR-T manufacturing involves the engineering with expensive GMP-grade viral vectors in centralized manufacturing facilities, whereas CIK cell production is successfully performed in local academic GMP facilities, and CIK cell treatment is now licensed in many countries. Moreover, the toxicities observed for CAR-T cells are not present in CIK cell-treated patients, thus further reducing the costs associated with hospitalization and post-infusion monitoring of patients, and ultimately encouraging the delivery of cell therapies in the outpatient setting. This review aims to give an overview of the limitations of CAR-T cell therapy and outline how the use of CIK cells could overcome such drawbacks thanks to their unique features. We highlight the undeniable advantages of using CIK cells as a therapeutic product, underlying the opportunity for further research on the topic.
Keywords: ATMP; CAR (chimeric antigen receptor) T cells; CIK (cytokine-induced killer) cells; GMP; GvHD; adoptive cell immunotherapy; hematological malignancies; solid tumors.
Copyright © 2023 Cappuzzello, Vigolo, D’Accardio, Astori, Rosato and Sommaggio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

