ADAMTS-7 Modulates Atherosclerotic Plaque Formation by Degradation of TIMP-1
- PMID: 37675562
- PMCID: PMC7615141
- DOI: 10.1161/CIRCRESAHA.123.322737
ADAMTS-7 Modulates Atherosclerotic Plaque Formation by Degradation of TIMP-1
Abstract
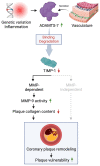
Background: The ADAMTS7 locus was genome-wide significantly associated with coronary artery disease. Lack of the ECM (extracellular matrix) protease ADAMTS-7 (A disintegrin and metalloproteinase-7) was shown to reduce atherosclerotic plaque formation. Here, we sought to identify molecular mechanisms and downstream targets of ADAMTS-7 mediating the risk of atherosclerosis.
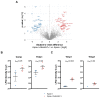
Methods: Targets of ADAMTS-7 were identified by high-resolution mass spectrometry of atherosclerotic plaques from Apoe-/- and Apoe-/-Adamts7-/- mice. ECM proteins were identified using solubility profiling. Putative targets were validated using immunofluorescence, in vitro degradation assays, coimmunoprecipitation, and Förster resonance energy transfer-based protein-protein interaction assays. ADAMTS7 expression was measured in fibrous caps of human carotid artery plaques.
Results: In humans, ADAMTS7 expression was higher in caps of unstable as compared to stable carotid plaques. Compared to Apoe-/- mice, atherosclerotic aortas of Apoe-/- mice lacking Adamts-7 (Apoe-/-Adamts7-/-) contained higher protein levels of Timp-1 (tissue inhibitor of metalloprotease-1). In coimmunoprecipitation experiments, the catalytic domain of ADAMTS-7 bound to TIMP-1, which was degraded in the presence of ADAMTS-7 in vitro. ADAMTS-7 reduced the inhibitory capacity of TIMP-1 at its canonical target MMP-9 (matrix metalloprotease-9). As a downstream mechanism, we investigated collagen content in plaques of Apoe-/- and Apoe-/-Adamts7-/- mice after a Western diet. Picrosirius red staining of the aortic root revealed less collagen as a readout of higher MMP-9 activity in Apoe-/- as compared to Apoe-/- Adamts7-/- mice. To facilitate high-throughput screening for ADAMTS-7 inhibitors with the aim of decreasing TIMP-1 degradation, we designed a Förster resonance energy transfer-based assay targeting the ADAMTS-7 catalytic site.
Conclusions: ADAMTS-7, which is induced in unstable atherosclerotic plaques, decreases TIMP-1 stability reducing its inhibitory effect on MMP-9, which is known to promote collagen degradation and is likewise associated with coronary artery disease. Disrupting the interaction of ADAMTS-7 and TIMP-1 might be a strategy to increase collagen content and plaque stability for the reduction of atherosclerosis-related events.
Keywords: aorta; atherosclerosis; disintegrins; mice; solubility.
Conflict of interest statement
Figures





References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. - PubMed
-
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. - PubMed
-
- Coronary Artery Disease (C4D) Genetics Consortium. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

