Study of Rivaroxaban for Cerebral Venous Thrombosis: A Randomized Controlled Feasibility Trial Comparing Anticoagulation With Rivaroxaban to Standard-of-Care in Symptomatic Cerebral Venous Thrombosis
- PMID: 37675613
- PMCID: PMC10615774
- DOI: 10.1161/STROKEAHA.123.044113
Study of Rivaroxaban for Cerebral Venous Thrombosis: A Randomized Controlled Feasibility Trial Comparing Anticoagulation With Rivaroxaban to Standard-of-Care in Symptomatic Cerebral Venous Thrombosis
Abstract
Background: Emerging data suggest that direct oral anticoagulants may be a suitable choice for anticoagulation for cerebral venous thrombosis (CVT). However, conducting high-quality trials in CVT is challenging as it is a rare disease with low rates of adverse outcomes such as major bleeding and functional dependence. To facilitate the design of future CVT trials, SECRET (Study of Rivaroxaban for Cerebral Venous Thrombosis) assessed (1) the feasibility of recruitment, (2) the safety of rivaroxaban compared with standard-of-care anticoagulation, and (3) patient-centered functional outcomes.
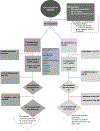
Methods: This was a phase II, prospective, open-label blinded-end point 1:1 randomized trial conducted at 12 Canadian centers. Participants were aged ≥18 years, within 14 days of a new diagnosis of symptomatic CVT, and suitable for oral anticoagulation; they were randomized to receive rivaroxaban 20 mg daily, or standard-of-care anticoagulation (warfarin, target international normalized ratio, 2.0-3.0, or low-molecular-weight heparin) for 180 days, with optional extension up to 365 days. Primary outcomes were annual rate of recruitment (feasibility); and a composite of symptomatic intracranial hemorrhage, major extracranial hemorrhage, or mortality at 180 days (safety). Secondary outcomes included recurrent venous thromboembolism, recanalization, clinically relevant nonmajor bleeding, and functional and patient-reported outcomes (modified Rankin Scale, quality of life, headache, mood, fatigue, and cognition) at days 180 and 365.
Results: Fifty-five participants were randomized. The rate of recruitment was 21.3 participants/year; 57% of eligible candidates consented. Median age was 48.0 years (interquartile range, 38.5-73.2); 66% were female. There was 1 primary event (symptomatic intracranial hemorrhage), 2 clinically relevant nonmajor bleeding events, and 1 recurrent CVT by day 180, all in the rivaroxaban group. All participants in both arms had at least partial recanalization by day 180. At enrollment, both groups on average reported reduced quality of life, low mood, fatigue, and headache with impaired cognitive performance. All metrics improved markedly by day 180.
Conclusions: Recruitment targets were reached, but many eligible participants declined randomization. There were numerically more bleeding events in patients taking rivaroxaban compared with control, but rates of bleeding and recurrent venous thromboembolism were low overall and in keeping with previous studies. Participants had symptoms affecting their well-being at enrollment but improved over time.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT03178864.
Keywords: cognition; hemorrhage; rare disease; rivaroxaban; warfarin.
Conflict of interest statement
Figures
References
-
- Zhou LW, Yu AYX, Ngo L, Hill MD, Field TS. Incidence of Cerebral Venous Thrombosis: A Population-Based Study, Systematic Review, and Meta-Analysis. Stroke. 2023;54:169–177. - PubMed
-
- Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. Int. J. Stroke. 2017;12:687–697. - PubMed
-
- Koopman K, Uyttenboogaart M, Vroomen PC, van der Meer J, De Keyser J, Luijckx G-J. Long-term sequelae after cerebral venous thrombosis in functionally independent patients. J. Stroke Cerebrovasc. Dis. 2009;18:198–202. - PubMed
-
- Hiltunen S, Putaala J, Haapaniemi E, Tatlisumak T. Long-term outcome after cerebral venous thrombosis: analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. J. Neurol. 2016;263:477–484. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical