Chronic psychosocial stress triggers microglial-/macrophage-induced inflammatory responses leading to neuronal dysfunction and depressive-related behavior
- PMID: 37675659
- PMCID: PMC10842267
- DOI: 10.1002/glia.24464
Chronic psychosocial stress triggers microglial-/macrophage-induced inflammatory responses leading to neuronal dysfunction and depressive-related behavior
Abstract
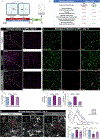
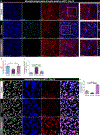
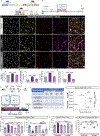
Chronic environmental stress and traumatic social experiences induce maladaptive behavioral changes and is a risk factor for major depressive disorder (MDD) and various anxiety-related psychiatric disorders. Clinical studies and animal models of chronic stress have reported that symptom severity is correlated with innate immune responses and upregulation of neuroinflammatory cytokine signaling in brain areas implicated in mood regulation (mPFC; medial Prefrontal Cortex). Despite increasing evidence implicating impairments of neuroplasticity and synaptic signaling deficits into the pathophysiology of stress-related mental disorders, how microglia may modulate neuronal homeostasis in response to chronic stress has not been defined. Here, using the repeated social defeat stress (RSDS) mouse model we demonstrate that microglial-induced inflammatory responses are regulating neuronal plasticity associated with psychosocial stress. Specifically, we show that chronic stress induces a rapid activation and proliferation of microglia as well as macrophage infiltration in the mPFC, and these processes are spatially related to neuronal activation. Moreover, we report a significant association of microglial inflammatory responses with susceptibility or resilience to chronic stress. In addition, we find that exposure to chronic stress exacerbates phagocytosis of synaptic elements and deficits in neuronal plasticity. Importantly, by utilizing two different CSF1R inhibitors (the brain penetrant PLX5622 and the non-penetrant PLX73086) we highlight a crucial role for microglia (and secondarily macrophages) in catalyzing the pathological manifestations linked to psychosocial stress in the mPFC and the resulting behavioral deficits usually associated with depression.
Keywords: chronic stress; depressive-related behavior; innate immunity; macrophages; microglia; neuroinflammation; neuronal dysfunction; susceptibility/resilience to stress; synaptic pruning.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Competing Interests statement
The authors declare no competing interests.
Figures



References
-
- Li Q. & Barres BA Microglia and macrophages in brain homeostasis and disease. Nature Reviews Immunology 18, 225 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

