Anti-CD20 monoclonal antibody therapy in postpartum women with neurological conditions
- PMID: 37675826
- PMCID: PMC10647007
- DOI: 10.1002/acn3.51893
Anti-CD20 monoclonal antibody therapy in postpartum women with neurological conditions
Abstract
Objective: Postpartum, patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) have increased risk for disease activity. Anti-CD20 IgG1 monoclonal antibodies (mAb) are increasingly used as disease-modifying therapies (DMTs). Patients may wish to both breastfeed and resume DMT postpartum. This study aimed to determine the transfer of anti-CD20 IgG1 mAbs, ocrelizumab, and rituximab (OCR/RTX), into mature breastmilk and describe maternal and infant outcomes.
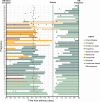
Methods: Fifty-seven cis-women receiving OCR/RTX after 59 pregnancies and their infants were enrolled and followed up to 12M postpartum or 90 days post-infusion. Breastmilk was collected pre-infusion and serially up to 90 days and assayed for mAb concentration. Medical records and patients' questionnaire responses were obtained to assess neurologic, breastfeeding, and infant development outcomes.
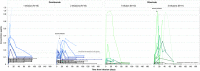
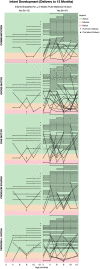
Results: The median average concentration of mAb in breastmilk was low (OCR: 0.08 μg/mL, range 0.05-0.4; RTX: 0.03 μg/mL, range 0.005-0.3). Concentration peaked 1-7 days post-infusion in most (77%) and was nearly undetectable after 90 days. Median average relative infant dose was <1% (OCR: 0.1%, range 0.07-0.7; RTX: 0.04%, range 0.005-0.3). Forty-three participants continued to breastfeed post-infusion. At 8-12 months, the proportion of infants' growth between the 3rd and 97th World Health Organization percentiles did not differ for breastfed (36/40) and non-breastfed (14/16, p > 0.05) infants; neither did the proportion with normal development (breastfed: 37/41, non-breastfed: 11/13; p > 0.05). After postpartum infusion, two mothers experienced a clinical relapse.
Interpretation: These confirm minimal transfer of mAb into breastmilk. Anti-CD20 mAb therapy stabilizes MS activity before conception to the postpartum period, and postpartum treatments appears to be safe and well-tolerated for both mother and infant.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
All authors’ financial disclosures are included in the attached ICMJE forms. The drugs, ocrelizumab and rituximab, are tested in this study. Genentech (a subsidiary of the Roche Group) is the manufacturer of ocrelizumab and co‐markets rituximab, a drug developed and co‐marketed by Biogen. Potential conflict of interest disclosures related to the study are outlined below: CB has received scientific advisory board fees and travel support from Genentech which manufactures and co‐markets the AIC reports speaker honoraria from Biogen which co‐markets a drug tested in this study. DJ has received research support and consulting/scientific advisory board fees from Biogen and Genentech which manufacture and co‐market drugs tested in this study. KMK is an advisory board member for Roche and has received grants from Roche which manufactures and co‐markets a drug tested in this study. KMK also reports support from an MS fellowship grant during her fellowship from Biogen, which co‐markets a drug tested in this study. EEL reports research support and consulting/advisory board fees from Genentech which manufactures and co‐markets the drugs tested in this study. PR reports consulting and/or speaking honoraria from Genentech which manufactures and co‐markets the drugs tested in this study. CSR reports consulting fees or honoraria from Genentech which manufactures and co‐markets the drugs tested in this study. TW has received honoraria for speaking and education activities through Biogen Idec which co‐markets a drug tested in this study. KH has received research support, consulting, speaking, and/or advisory board fees from Roche and Biogen which manufacture and co‐market the drugs tested in this study. RB reports research support from Biogen and Roche‐Genentech which manufactures and co‐markets the drugs tested in this study.
Figures