Sexual and drug use risk behaviour trajectories among people treated for recent HCV infection: the REACT study
- PMID: 37675828
- PMCID: PMC10483502
- DOI: 10.1002/jia2.26168
Sexual and drug use risk behaviour trajectories among people treated for recent HCV infection: the REACT study
Abstract
Introduction: Exploration of sexual and drug use behaviours following treatment for recent hepatitis C virus (HCV) is limited. This analysis modelled behavioural trajectories following treatment for recent HCV and assessed reinfection.
Methods: Participants treated for recent HCV in an international trial (enrolled 2017-2019) were followed at 3-monthly intervals for up to 2 years to assess longitudinal behaviours. Population-averaged changes were assessed using generalized estimating equations. Distinct behavioural trajectories were identified using group-based trajectory modelling. HCV reinfection incidence was calculated using person-years (PY) of observation.
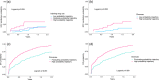
Results: During the follow-up of 212 participants (84% gay and bisexual men [GBM]; 69% HIV; 26% current injecting drug use [IDU]), behavioural trajectories for IDU and stimulant use (past month) did not change. However, population-averaged decreases in the likelihood of daily IDU (adjusted odds ratio [AOR] 0.83; 95% CI 0.72, 0.95) and opioid use (AOR 0.84; 95% CI 0.75, 0.93) were observed. Among GBM, behavioural trajectories for chemsex did not change. Population-averaged decreases in condomless anal intercourse with casual male partners (CAI-CMP) (AOR 0.95; 95% CI 0.90, 0.99) and group-sex (AOR 0.86; 95% CI 0.80, 0.93) were observed, but masked distinct trajectories. While a proportion had a decreased probability of CAI-CMP (23%) and group-sex (59%) post-treatment, a substantial proportion retained a high probability of these behaviours. High HCV reinfection incidence was observed for the sustained high probability IDU (33.0/100 PY; 95% CI 17.7, 61.3) and chemsex (23.3/100 PY; 95% CI 14.5, 37.5) trajectories.
Conclusions: Limited sexual and drug use behavioural change was observed following treatment for recent HCV, supporting access to surveillance and (re)treatment.
Trial registration: ClinicalTrials.gov NCT02625909.
Keywords: GBM; HCV; HIV; PWID; STI; reinfection.
© 2023 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Dore GJ, Martinello M, Alavi M, Grebely J. Global elimination of hepatitis C virus by 2030: why not? Nat Med. 2020;. 26(2):157–60. - PubMed
-
- Blach S, Zeuzem S, Manns M, Altraif I, Duberg AS, Muljono DH, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;. 2(3):161–76. - PubMed
-
- Blach S, Terrault NA, Tacke F, Gamkrelidze I, Craxi A, Tanaka J, et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;. 1253(21):1–21. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

