Full eradication of pre-clinical human papilloma virus-induced tumors by a lentiviral vaccine
- PMID: 37675835
- PMCID: PMC10565635
- DOI: 10.15252/emmm.202317723
Full eradication of pre-clinical human papilloma virus-induced tumors by a lentiviral vaccine
Abstract
Human papillomavirus (HPV) infections are the cause of all cervical and numerous oropharyngeal and anogenital cancers. The currently available HPV vaccines, which induce neutralizing antibodies, have no therapeutic effect on established tumors. Here, we developed an immuno-oncotherapy against HPV-induced tumors based on a non-integrative lentiviral vector encoding detoxified forms of the Early E6 and E7 oncoproteins of HPV16 and 18 genotypes, namely, "Lenti-HPV-07". A single intramuscular injection of Lenti-HPV-07 into mice bearing established HPV-induced tumors resulted in complete tumor eradication in 100% of the animals and was also effective against lung metastases. This effect correlated with CD8+ T-cell induction and profound remodeling of the tumor microenvironment. In the intra-tumoral infiltrates of vaccinated mice, the presence of large amounts of activated effector, resident memory, and transcription factor T cell factor-1 (TCF-1)+ "stem-like" CD8+ T cells was associated with full tumor eradication. The Lenti-HPV-07-induced immunity was long-lasting and prevented tumor growth after a late re-challenge, mimicking tumor relapse. Lenti-HPV-07 therapy synergizes with an anti-checkpoint inhibitory treatment and therefore shows promise as an immuno-oncotherapy against established HPV-mediated malignancies.
A lentiviral vector‐based immuno‐oncotherapy, used in mice bearing Human PapillomaVirus (HPV)‐induced tumors, triggers a T‐cell immunity able to fully eradicate tumors in 100% of experimental animals. A single intramuscular injection of this vector deeply remodels the tumor microenvironment and favors the actions of anti‐tumor immune effectors.
The available prophylactic HPV vaccines mainly induce neutralizing antibodies which prevent viral infection but have no therapeutic effect on HPV‐induced tumors.
A lentiviral vector‐based immuno‐oncotherapy (Lenti‐HPV‐07) encodes antigens from HPV16 and HPV18 genotypes.
A single administration of Lenti‐HPV‐07 is enough to eradicate small or large HPV‐induced tumors in 100% of animals in a preclinical model.
Lenti‐HPV‐07 treatment induces a long‐term protective immunity which avoids tumor relapse.
Lenti‐HPV‐07 immuno‐oncotherapy can be combined to other immunotherapies to potentiate their actions.
Keywords: early E6-E7 oncoproteins; immuno-oncotherapy; intra-tumoral immune cells; lentiviral vector; tumor microenvironment.
© 2023 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
LD, IF, BV, FLC, FM, PA, T‐MN, AN, FN, FA, LM, and PC declare competing financial interests related to the publication of this study. PC is the founder and CSO of TheraVectys. LD, IF, BV, FLC, FM, PA, T‐MN, AN, FN, and FA are employees of TheraVectys. LM has a consultancy activity for TheraVectys. LD, IF, FM, AN, FA, LM, and PC are inventors of a pending patent directed to the potential of Lenti‐HPV‐07 vaccination against HPV‐induced cancers. Other authors declare no competing interests.
Figures
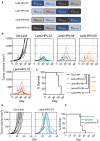
- A
Four antigenic designs of non‐oncogenic, inactivated forms of E6 and E7 antigens from HPV16 and HPV18 with distinct permutations, as encoded by non‐integrative LV‐based HPV therapeutic vaccine candidates.
- B, C
C57BL/6 mice (n = 8/group) were engrafted s.c. on the right flank with 1 × 106 TC‐1 cells. At day 10 post‐engraftment, when the tumor volume reached an average of 70 mm3, mice were randomized. Vaccination was performed at day 11 by i.m. injection of 1 × 109 TU/mouse of individual Lenti‐HPV vectors. Tumor‐bearing control animals received Ctrl Lenti. (B) Spaghetti plots of tumor size over time. The gray arrows indicate the time point at which the vaccine was injected. (C) Survival curve of the animals.
- D, E
C57BL/6 mice (n = 7–8/group) were engrafted s.c. on the right flank with 1 × 106 TC‐1 cells. At day 20, when the tumor volume reached an average of 450 mm3, mice were randomized. Vaccination was performed at day 20 by i.m. injection of 1 × 109 TU/mouse of Lenti‐HPV‐07. Tumor‐bearing control animals received Ctrl Lenti. (D) Spaghetti plots of tumor size over time. The gray arrows indicate the time point at which the vaccine was injected. (E) Survival curve of the animals.


- A
C57BL/6 mice received i.m.: (1) 1 × 109 TU of Ctrl Lenti (n = 3), (2) 1 × 108 TU (n = 14) of Lenti‐HPV‐07, or (3) 1 × 109 TU (n = 8) of Lenti‐HPV‐07. At day 14 post injection, splenocytes from individual mice were studied by IFN‐γ ELISPOT after in vitro stimulation with pools of synthetic 15‐mers spanning the sequence of E6HPV16, E6HPV18, E7HPV16, or E7HPV18.
- B–D
T‐cell cytokine responses of splenocytes from Ctrl Lenti‐ or Lenti‐HPV‐07‐injected mice (n = 5) were also studied by ICS, with or without stimulation with a mixture of E7HPV16:ETTDPD
RAHYNIVTF and E7HPV16:PDRAHYNIVTF CCKC peptides, both containing the RAHYNIVTF H‐2Db‐restricted T‐cell epitope. Bold characters represent H‐2Db anchor residues. (B) Cytometric gating strategy carried out on IL‐2‐, TNF‐α‐, and IFN‐γ‐producing CD8+ T cells. (C) Recapitulative frequencies of each (poly)functional cell subsets and (D) degranulation activity of the IFN‐γ‐producing CD8+ T cells, assessed by surface CD107a staining. - E
HHD‐DR1 mice received i.m.: (1) 1 × 109 TU of a Ctrl Lenti (n = 4), (2) 1 × 108 TU (n = 4) Lenti‐HPV‐07, or (3) 1 × 109 TU (n = 5) of Lenti‐HPV‐07 and their splenocytes were studied by IFN‐γ ELISPOT, as described in (A).
- F
Mice (n = 7/group) engrafted s.c. with 1 × 106 tumor cells were injected at day 14 with 1 × 109 TU of Ctrl Lenti or Lenti‐HPV‐07 and then treated with a Ctrl Ig or anti‐CD4 or anti‐CD8 mAbs, as detailed in Material and Methods. Spaghetti plots of tumor growth in the various group. The gray arrows indicate the time point at which Lenti‐HPV‐07 was injected.
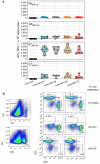
- A
Comparable immunogenicity of various Lenti‐HPV. C57BL/6 mice (n = 5/group) were immunized i.m. at day 0 with Ctrl Lenti or each of the Lenti‐HPV. On day 14, T splenocyte responses were assessed by IFN‐γ ELISPOT after in vitro stimulation with a pool of 15‐mer peptides spanning the sequence of E6HPV16, E6HPV18, E7HPV16 or E7HPV18, as detailed in Fig 2A. Comparable T‐cell immunogenicity of the four Lenti‐HPVs showed that the position of the antigen in the four poly‐antigens encoded by these vectors has no effect on their immunogenicity.
- B
Efficacy of anti‐CD4 or anti‐CD8 depletion in vivo. Tumor‐free C57BL/6 mice (n = 2/group) received two i.p. injections of 250 μg anti‐CD4 (clone GK1.5), anti‐CD8 (clone H35.17.2) or a control Ig isotype 3 days apart. On day 4 after the second injection, the spleens from individual mice were assessed by anti‐CD3, anti‐CD4, and anti‐CD8 mAb staining and cytometry analysis to evaluate the efficacy of T‐subset depletion. The results from two individual mice are shown. Note that the mAbs used for depletion and cytometric studies were different to allow the distinction between epitope masking and genuine T‐subset depletion.

- A, B
Representative dot blots of tumor infiltrating T cells, studied by cytometry.
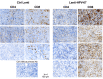
- C
Representative tumor infiltrating T cells, studied by anti‐CD8 immunohistochemistry.
- D–H
Representative dot blots of tumor infiltrating T cells, studied by cytometry. For cytometry data, the percentage of each subset was compared between the two groups and statistical significance was determined using two‐tailed unpaired t‐tests (**P ≤ 0.01, ***P ≤ 0.001).
- I
PCA projection plot of the parameters studied in (A, B, D–H). Differential characteristics of intra‐tumoral T cells between Ctrl Lenti‐ and Lenti‐HPV‐07‐treated groups. PC1 of 10 variables, shown in (A, B, D–H) split the samples by treatment. Proportion of variance for PC1 = 87.71%.

- A–C
The tumor‐engrafted and vaccinated mice are those detailed in Fig 3 (n = 7/group). Tumor infiltrating innate immune cells in Lenti Ctrl‐ or Lenti‐HPV‐07‐injected mice were studied on day 11 post‐vaccination by cytometry. Representative blots for various innate cells are shown. The percentage of each subset was compared between the two groups and statistical significance determined using two‐tailed unpaired t tests (ns: non‐significant, ***P ≤ 0.001).
- D
PCA projection plot of the parameters studied in (A–C). Differential characteristics of intra‐tumoral innate immune cells between Ctrl Lenti‐ and Lenti‐HPV‐07‐treated groups. PC1 of three variables, shown in (A–C), split the samples by treatment. Proportion of variance for PC1 = 93.48%.
- E
Ratio of intra‐tumoral T cells vs CD11b+ cells. Statistical significance was determined using two‐tailed unpaired t tests (***P ≤ 0.001).
- F
Comparative expression of PD‐L1 at the surface of FSChi CD45− tumor cells, shown as overlayed cytometric histograms for individual mice from the two groups. Statistical significance was determined using two‐tailed unpaired t tests (***P ≤ 0.001).
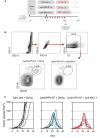
- A
Timeline of the tumor engraftment, Lenti‐HPV‐07 treatment and anti‐NK1.1 mAb injection. C57BL/6 mice (n = 7/group) were engrafted s.c. on the flank with 1 × 106 tumor cells. On day 14 post‐engraftment, mice were injected with 1 × 109 TU of Ctrl Lenti or Lenti‐HPV‐07. From day 13 to 30 mice were treated with a control Ig or anti‐NK1.1 mAb by 9 successive i.p. injection of 300 μg/mouse/injection.
- B
Efficacy of anti‐NK1.1 depletion in vivo was verified on the splenoctyes of one mouse per group assessed by anti‐CD11b and anti‐NKp46 mAb staining and cytometry analysis. Note that the mAbs used for NK cell depletion and cytometric studies target distinct markers to ensure genuine NK cell depletion and not epitope masking.
- C
Spaghettis plots of tumor growth in the mice.
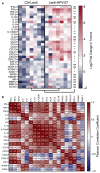
- A
The heatmap represents the log2 fold change in mRNA expression in the total tumors of mice (n = 7/group) engrafted with TC‐1 on day 0, vaccinated with Lenti‐HPV‐07 on day 13, and removed for qRT‐PCR analysis of 33 analytes on day 22. For each sample, the mRNA abundance (C T value) of the target genes was normalized to that of the endogenous β‐globin reference gene and compared to a calibrator value corresponding to the mean of the C T values determined in the tumors from the Ctrl Lenti group. The fold change in gene expression was further calculated using . Statistical significance was evaluated using the Mann–Whitney tests (ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001).
- B
Pearson correlation coefficient of differentially expressed analytes in Lenti‐HPV‐vaccinated mice. Col1A1, collagen, type 1, alpha 1; FGF, fibroblast growth factor; MMP, matrix metalloproteinase.
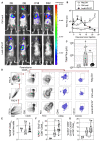
- A
C57BL/6 mice were injected i.v. with 1.5 × 105 nLuc‐TC‐1 cells. On day 5, mice received a single i.m. injection of 1 × 109 TU of Ctrl Lenti or Lenti‐HPV‐07 (n = 11/group). The development of pulmonary metastatic foci was monitored by bioluminescence imaging. The luminescence values of the regions where signals were detectable, that is, thoracic region, were evaluated as the total flux of photons/s (p/s) right after furimazine Z108 substrate administration. The baseline signal was obtained from mice injected with neither nLuc‐TC‐1 cells nor LVs but injected with the same amounts of furimazine. Representative images obtained from nLuc‐TC‐1‐injected and Ctrl Lenti‐ or Lenti‐HPV‐07‐treated mice at various time points post‐tumor engraftment.
- B
Follow‐up of total flux over time (n = 11/group), shown as the mean ± SEM of biological replicates. The black arrow indicates the time point at which Ctrl Lenti or Lenti‐HPV‐07 were injected. Dotted line represents the basal total flux. Statistical significance was determined using two‐tailed unpaired t‐tests (ns, not significant, **P < 0.01).
- C
The total flux for individual mice of the three experimental groups on day 22 post‐tumor engraftment. Statistical significance was determined using two‐tailed unpaired t tests (ns, not significant, **P < 0.01).
- D
At day 15 post‐nLuc‐TC‐1 i.v. instillation, groups of untreated mice (Neg Ctrl) (n = 4) or Lenti Ctrl‐ (n = 10) or Lenti‐HPV‐07‐ (n = 10) treated and tumor bearing mice received an i.v. injection of PE‐anti‐CD45 mAb, 3 min before sacrifice. This in vivo staining allows cytometrical distinction of lung parenchymal (CD45i.v.−) from vascular (CD45i.v.+) hematopoietic cells. Live CD8+ CD45i.v.− T cells were gated and analyzed for their expression of various markers. A representative blot for each group is shown.
- E–G
Percentages of each subset are compared between the groups. Statistical significance was evaluated using the Mann–Whitney tests (ns, not significant, *P < 0.05, **P < 0.01).
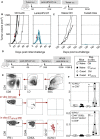
- A
Timeline of s.c. tumor engraftment and Lenti‐HPV‐07 vaccination. C57BL/6 mice (n = 7) were engrafted on the flank with 1 × 106 tumor cells. On day 18 post‐engraftment, when the tumor volume reached an average of 200 mm3, mice were randomized. Lenti‐HPV‐07 vaccination was performed on day 18. Tumor‐bearing negative control animals received Ctrl Lenti.
- B
Spaghetti plots of tumor growth. The red arrows indicate the time point at which Ctrl Lenti or Lenti‐HPV‐07 were injected.
- C
On day 120, cured mice were engrafted s.c. with 1 × 106 tumor cells on the opposite flank and left untreated to mimic tumor relapse. Age‐matched naïve control mice (n = 4) showed the growth capacity of the engrafted tumor cells used in the relapse part of the experiment.
- D–F
In an independent experiment, Lenti‐HPV‐treated and cured mice which have been re‐challenged (n = 5/group), received at day 286 post tumor engraftment an i.v. injection of an adjuvant‐free mixture of E7HPV16:ETTDPD
RAHYNIVTF and E7HPV16:PDRAHYNIVTF CCKC peptides (25 μg of each/mouse). Naïve control mice received the same i.v. treatment. At 48 h post injection, splenocytes were stimulated in vitro with homologous or negative control peptides during 6 h prior to IFN‐γ ICS and surface labeling. (E) Gating strategy and representative blots. (F) Percentages of IFN‐γ+ versus total CD8+ T cells (top) and those of IFN‐γ+ CD44+ CD62L− CD127+ KLRG1− (memory precursor cells) (bottom) versus total CD8+ T cells. Statistical significance was determined by the Mann–Whitney t‐test (*P < 0.05, **P < 0.01).
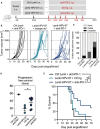
- A
Timeline of tumor s.c. engraftment and combinatory Lenti‐HPV‐07 and anti‐PD1 mAb treatment. C57BL/6 mice (n = 10–14/group) were engrafted on the flank with 1 × 106 tumor cells. On day 13 post‐engraftment, when the tumor volume reached an average of 120–140 mm3, mice were treated with the suboptimal dose of 1 × 108 TU of Ctrl Lenti or Lenti‐HPV‐07. Mice were then treated 2–3 times a week with anti‐PD1 mAb for a total of six injections from days 17 to 31.
- B
Spaghetti plots of tumor growth. The gray arrows indicate the time point at which Ctrl Lenti or Lenti‐HPV‐07 were injected.
- C
Percentages of mice, without, with partial or complete antitumor response.
- D
Progression‐free survival (PFS) time of mice bearing TC‐1 tumors treated with suboptimal dose of Lenti‐HPV07 and anti‐PD‐1. PFS described the duration of response from the day that tumor reaches maximum size until the day that tumor exceeds maximum size again in responsive mice (n = 10–14/group). Statistical significance was determined using two‐tailed unpaired t tests (*P < 0.05).
- E
Survival curves of animals (n = 10–14/group), followed for 85 days. Statistical significance was determined using Log‐rank Mantel‐Cox tests, (*P = 0.0159, **P = 0.071). Mice were sacrificed when the size of the tumors reached 1,500 mm3, in accordance with the defined humane endpoints.
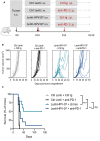
- A
Timeline of the tumor engraftment and combinatory treatment with Lenti‐HPV‐07 vaccination and anti‐PD1 injections. C57BL/6 mice (n = 10/group) were engrafted s.c. on the flank with 1 × 106 tumor cells. On day 10 post‐engraftment, when the tumor volume reached an average of 100–120 mm3, mice were injected with a suboptimal dose of 1 × 108 TU Ctrl Lenti or Lenti‐HPV‐07. Mice were treated with anti‐PD1 mAb on the same day and on day 13.
- B
Spaghettis plots of tumor growth in the mice. The gray arrows indicate the time point at which the vaccine was injected.
- C
Survival curves of the animals (n = 10/group) shown in (B) followed for 130 days. Statistical significance was determined by Log‐rank Mantel‐Cox tests (ns, not significant, **P ≤ 0.01, ***P ≤ 0.001). Note that there is a net tendency to decline in the survival of mice treated with Lenti‐HPV‐07 + anti‐PD1 compared to the mice treated with Lenti‐HPV‐07 alone, even though this difference did not reach statistical significance. Mice were sacrificed when the size of the tumors reached 1,500 mm3, in accordance with the defined humane endpoints.
References
-
- Adotevi O, Mollier K, Neuveut C, Dosset M, Ravel P, Fridman WH, Tartour E, Charneau P, Wain‐Hobson S, Langlade‐Demoyen P (2010) Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8 T‐cell immunity in vivo . Blood 115: 3025–3032 - PubMed
-
- Albershardt TC, Parsons AJ, Reeves RS, Flynn PA, Campbell DJ, Ter Meulen J, Berglund P (2020) Therapeutic efficacy of PD1/PDL1 blockade in B16 melanoma is greatly enhanced by immunization with dendritic cell‐targeting lentiviral vector and protein vaccine. Vaccine 38: 3369–3377 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

