Translation of dipeptide repeat proteins in C9ORF72 ALS/FTD through unique and redundant AUG initiation codons
- PMID: 37675986
- PMCID: PMC10541178
- DOI: 10.7554/eLife.83189
Translation of dipeptide repeat proteins in C9ORF72 ALS/FTD through unique and redundant AUG initiation codons
Abstract
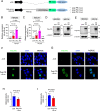
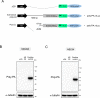
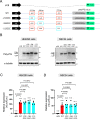
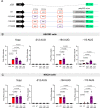
A hexanucleotide repeat expansion in C9ORF72 is the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). A hallmark of ALS/FTD pathology is the presence of dipeptide repeat (DPR) proteins, produced from both sense GGGGCC (poly-GA, poly-GP, poly-GR) and antisense CCCCGG (poly-PR, poly-PG, poly-PA) transcripts. Translation of sense DPRs, such as poly-GA and poly-GR, depends on non-canonical (non-AUG) initiation codons. Here, we provide evidence for canonical AUG-dependent translation of two antisense DPRs, poly-PR and poly-PG. A single AUG is required for synthesis of poly-PR, one of the most toxic DPRs. Unexpectedly, we found redundancy between three AUG codons necessary for poly-PG translation. Further, the eukaryotic translation initiation factor 2D (EIF2D), which was previously implicated in sense DPR synthesis, is not required for AUG-dependent poly-PR or poly-PG translation, suggesting that distinct translation initiation factors control DPR synthesis from sense and antisense transcripts. Our findings on DPR synthesis from the C9ORF72 locus may be broadly applicable to many other nucleotide repeat expansion disorders.
Keywords: AUG initiation codons; C9ORF72; amyotrophic lateral sclerosis; dipeptide protein repeats; frontotemporal dementia; genetics; genomics; human; iPSC-derived neurons; mouse; neuroscience.
© 2023, Sonobe et al.
Conflict of interest statement
YS, SL, GK, FG, RR No competing interests declared, YG, DK affiliated with Biogen. The authors have no financial interests to declare, PK Reviewing editor, eLife
Figures









Update of
- doi: 10.1101/2022.08.06.503063
Similar articles
-
Repeated repeat problems: Combinatorial effect of C9orf72-derived dipeptide repeat proteins.Int J Biol Macromol. 2019 Apr 15;127:136-145. doi: 10.1016/j.ijbiomac.2019.01.035. Epub 2019 Jan 9. Int J Biol Macromol. 2019. PMID: 30639592
-
Chimeric Peptide Species Contribute to Divergent Dipeptide Repeat Pathology in c9ALS/FTD and SCA36.Neuron. 2020 Jul 22;107(2):292-305.e6. doi: 10.1016/j.neuron.2020.04.011. Epub 2020 May 5. Neuron. 2020. PMID: 32375063 Free PMC article.
-
A C. elegans model of C9orf72-associated ALS/FTD uncovers a conserved role for eIF2D in RAN translation.Nat Commun. 2021 Oct 15;12(1):6025. doi: 10.1038/s41467-021-26303-x. Nat Commun. 2021. PMID: 34654821 Free PMC article.
-
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels.Autophagy. 2021 Nov;17(11):3306-3322. doi: 10.1080/15548627.2021.1872189. Epub 2021 Feb 26. Autophagy. 2021. PMID: 33632058 Free PMC article. Review.
-
Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD.Front Cell Neurosci. 2021 Feb 18;15:637548. doi: 10.3389/fncel.2021.637548. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33679328 Free PMC article. Review.
Cited by
-
Frontotemporal-TDP and LATE Neurocognitive Disorders: A Pathophysiological and Genetic Approach.Brain Sci. 2023 Oct 18;13(10):1474. doi: 10.3390/brainsci13101474. Brain Sci. 2023. PMID: 37891841 Free PMC article. Review.
-
Translation dysregulation in neurodegenerative diseases: a focus on ALS.Mol Neurodegener. 2023 Aug 25;18(1):58. doi: 10.1186/s13024-023-00642-3. Mol Neurodegener. 2023. PMID: 37626421 Free PMC article. Review.
-
Membraneless organelles in health and disease: exploring the molecular basis, physiological roles and pathological implications.Signal Transduct Target Ther. 2024 Nov 18;9(1):305. doi: 10.1038/s41392-024-02013-w. Signal Transduct Target Ther. 2024. PMID: 39551864 Free PMC article. Review.
-
Therapeutic Approaches for C9ORF72-Related ALS: Current Strategies and Future Horizons.Int J Mol Sci. 2025 Jun 28;26(13):6268. doi: 10.3390/ijms26136268. Int J Mol Sci. 2025. PMID: 40650046 Free PMC article. Review.
References
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao F-B. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathologica. 2013;126:385–399. doi: 10.1007/s00401-013-1149-y. - DOI - PMC - PubMed
-
- Ayhan F, Perez BA, Shorrock HK, Zu T, Banez-Coronel M, Reid T, Furuya H, Clark HB, Troncoso JC, Ross CA, Subramony SH, Ashizawa T, Wang ET, Yachnis AT, Ranum LP. SCA8 RAN polySer protein preferentially accumulates in white matter regions and is regulated by eIF3F. The EMBO Journal. 2018;37:e99023. doi: 10.15252/embj.201899023. - DOI - PMC - PubMed
-
- Banerjee P, Mehta AR, Nirujogi RS, Cooper J, James OG, Nanda J, Longden J, Burr K, McDade K, Salzinger A, Paza E, Newton J, Story D, Pal S, Smith C, Alessi DR, Selvaraj BT, Priller J, Chandran S. Cell-autonomous immune dysfunction driven by disrupted autophagy in C9orf72-ALS iPSC-derived microglia contributes to neurodegeneration. Science Advances. 2023;9:eabq0651. doi: 10.1126/sciadv.abq0651. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

