A risk-based subgroup analysis of the effect of adjuvant S-1 in estrogen receptor-positive, HER2-negative early breast cancer
- PMID: 37676450
- PMCID: PMC10564670
- DOI: 10.1007/s10549-023-07099-4
A risk-based subgroup analysis of the effect of adjuvant S-1 in estrogen receptor-positive, HER2-negative early breast cancer
Abstract
Purpose: The Phase III POTENT trial demonstrated the efficacy of adding S-1 to adjuvant endocrine therapy for estrogen receptor-positive, HER2-negative early breast cancer. We investigated the efficacy of S-1 across different recurrence risk subgroups.
Methods: This was a post-hoc exploratory analysis of the POTENT trial. Patients in the endocrine-therapy-only arm were divided into three groups based on composite risk values calculated from multiple prognostic factors. The effects of S-1 were estimated using the Cox model in each risk group. The treatment effects of S-1 in patients meeting the eligibility criteria of the monarchE trial were also estimated.
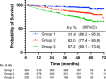
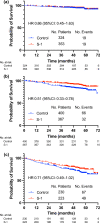
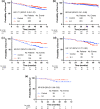
Results: A total of 1,897 patients were divided into three groups: group 1 (≤ lower quartile of the composite values) (N = 677), group 2 (interquartile range) (N = 767), and group 3 (> upper quartile) (N = 453). The addition of S-1 to endocrine therapy resulted in 49% (HR: 0.51, 95% CI: 0.33-0.78) and 29% (HR: 0.71, 95% CI 0.49-1.02) reductions in invasive disease-free survival (iDFS) events in groups 2 and 3, respectively. We could not identify any benefit from the addition of S-1 in group 1. The addition of S-1 showed an improvement in iDFS in patients with one to three positive nodes meeting the monarchE cohort 1 criteria (N = 290) (HR: 0.47, 95% CI: 0.29-0.74).
Conclusions: The benefit of adding adjuvant S-1 was particularly marked in group 2. Further investigations are warranted to explore the optimal usage of adjuvant S-1.
Keywords: Adjuvant; Breast neoplasms; Chemotherapy; Drug therapy; Estrogen; Receptors; Recurrence.
© 2023. The Author(s).
Conflict of interest statement
MTa received grants from Taiho (Tokyo, Japan) during the conduct of the study and has received grants from AstraZeneca (Osaka, Japan), Daiichi Sankyo (Tokyo, Japan), Eisai (Tokyo, Japan), Yakult (Tokyo, Japan), Medbis (Kyoto, Japan), the Japan Breast Cancer Research Group Association (JBCRG), the Kyoto Breast Cancer Research Network (KBCRN), ABCSG, and IQVIA Japan; and personal fees from Chugai (Tokyo, Japan), AstraZeneca, Daiichi Sankyo, Taiho, Pfizer (Tokyo, Japan), Eli Lilly (Kobe, Japan), Eisai, and MSD (Tokyo, Japan). HIw has received consulting fees from Daiichi Sankyo, Chugai, Lilly, AstraZeneca, Pfizer, MSD, and Novartis; and received personal fees from Daiichi Sankyo, Chugai, AstraZeneca, Lilly, MSD, Pfizer, and Taiho. NM has received grants from Chugai, AstraZeneca, MSD, Pfizer, Eli Lilly, Kyowa Kirin, Eisai, Novartis, Sanofi (Tokyo, Japan), Daiichi Sankyo, and Nippon Kayaku; and personal fees from Chugai, AstraZeneca, Pfizer, Elli Lilly, Eisai, and Takeda. NM is also a member of the board of directors of the Japan Breast Cancer Society (JBCS) and JBCRG. HM has received grants from Daiichi Sankyo, and Eisai; and personal fees from Taiho, Daiichi Sankyo, and Takeda. SS has received grants from AstraZeneca, Chugai, Daiichi Sankyo, MSD, and Taiho; and personal fees from AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa Kirin, MSD, Novartis, Ono (Osaka, Japan), Pfizer, Taiho, and Takeda. SS also received advisory fees from AstraZeneca, Chugai, Daiichi Sankyo, MSD, and Pfizer. TSa has received grants from Maruho, LabCorp Japan, Sanofi, Takeda, Novartis, MSD, Sawai, Covance Japan, Taiho, WJOG, Chugai, Nippon Kayaku, AstraZeneca, Eisai, Kyowa Kirin, Daiichi Sankyo, and Eli Lilly; and personal fees from Ono, Kyowa Kirin, Chugai, ASKA, Novartis, AstraZeneca, Eisai, Taiho, Takeda, Eli Lilly, Pfizer, MiRTeL, and Meiji Seika. KA has received personal fees from Chugai, Eisai, AstraZeneca, Taiho, Daiichi Sankyo, Pfizer, and Eli Lilly; and research grant from Chugai, Eisai, and Takeda. TSu has received research grants from Chugai, Eisai, and Kyoto Bridge. For Breakthrough Medicine (KBBM); and personal fees from Chugai, MSD, AstraZeneca, Pfizer, Eli Lillym Taiho, Daiichi Sankyo, and Eisai. TU has received personal fees from Chugai, Eisai, AstraZeneca, and Novartis; and research grant from Eli lilly. SO has received personal fees from Chugai, MSD, Eli Lilly, and Nippon Kayaku. HI has received personal fees from JMS, Eisai, Pfizer, Chugai, Kyowa Kirin, and Daiichi Sankyo; and research grant from Eisai, Daiichi Sankyo, Chugai, Nipro, and Takeda. MTo received grants from Taiho during the conduct of the study and has received grants from Chugai, Takeda, Pfizer, Taiho, JBCRG, KBCRN, Eisai, Eli Lilly, Daiichi Sankyo, AstraZeneca, Astellas (Tokyo, Japan), Shimadzu (Kyoto, Japan), Yakult, Nippon Kayaku, AFI technology, Luxonus (Kawasaki, Japan), Shionogi, GL Science, and Sanwa Shurui; personal fees from Chugai, Takeda, Pfizer, Kyowa Kirin, Taiho, Eisai, Daiichi Sankyo, AstraZeneca, Eli Lilly, MSD, Exact Science (Tokyo, Japan), Novartis, Shimadzu, Yakult, Nippon Kayaku, Devicore and Sysmex; and advisory fees from Daiichi Sankyo, Eli Lilly, BMS (Tokyo, Japan), Athenex Oncology, Bertis, Terumo (Tokyo, Japan), and Kansai Medical Net. MTo is also a member of the board of directors of JBCRG, KBCRN, and Organisation for Oncology and Translational Research. The other authors declare no other potential conflicts of interest.
Figures
References
-
- Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein LJ, Chia SKL, Dhesy-Thind S, Rastogi P, Alba E, Delaloge S, Martin M, Kelly CM, Ruiz-Borrego M, Gil-Gil M, Arce-Salinas CH, Brain EGC, Lee ES, Pierga JY, Bermejo B, Ramos-Vazquez M, Jung KH, Ferrero JM, Schott AF, Shak S, Sharma P, Lew DL, Miao J, Tripathy D, Pusztai L, Hortobagyi GN. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385:2336–2347. doi: 10.1056/NEJMoa2108873. - DOI - PMC - PubMed
-
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Goetz MP, Olson JA, Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW., Jr Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. - DOI - PMC - PubMed
-
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE, Jr, Dees EC, Perez EA, Olson JA, Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. - DOI - PMC - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/s0140-6736(11)61625-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

