Early Access to Testosterone Therapy in Transgender and Gender-Diverse Adults Seeking Masculinization: A Randomized Clinical Trial
- PMID: 37676662
- PMCID: PMC10485726
- DOI: 10.1001/jamanetworkopen.2023.31919
Early Access to Testosterone Therapy in Transgender and Gender-Diverse Adults Seeking Masculinization: A Randomized Clinical Trial
Abstract
Importance: Testosterone treatment is a necessary component of care for some transgender and gender-diverse individuals. Observational studies have reported associations between commencement of gender-affirming hormone therapy and improvements in gender dysphoria and depression, but there is a lack of data from randomized clinical trials.
Objective: To assess the effect of testosterone therapy compared with no treatment on gender dysphoria, depression, and suicidality in transgender and gender-diverse adults seeking masculinization.
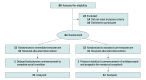
Design, setting, and participants: A 3-month open-label randomized clinical trial was conducted at endocrinology outpatient clinics and primary care clinics specializing in transgender and gender-diverse health in Melbourne, Australia, from November 1, 2021, to July 22, 2022. Participants included transgender and gender-diverse adults aged 18 to 70 years seeking initiation of testosterone therapy.
Interventions: Immediate initiation of testosterone commencement (intervention group) or no treatment (standard care waiting list of 3 months before commencement). This design ensured no individuals would be waiting longer than the time to standard care.
Main outcomes and measures: The primary outcome was gender dysphoria, as measured by the Gender Preoccupation and Stability Questionnaire. Secondary outcomes included the Patient Health Questionnaire-9 (PHQ-9) to assess depression and the Suicidal Ideation Attributes Scale (SIDAS) to assess suicidality. Questionnaires were undertaken at 0 and 3 months. The evaluable cohort was analyzed.
Results: Sixty-four transgender and gender-diverse adults (median [IQR] age, 22.5 [20-27] years) were randomized. Compared with standard care, the intervention group had a decrease in gender dysphoria (mean difference, -7.2 points; 95% CI, -8.3 to -6.1 points; P < .001), a clinically significant decrease in depression (ie, change in score of 5 points on PHQ-9; mean difference, -5.6 points; 95% CI, -6.8 to -4.4 points; P < .001), and a significant decrease in suicidality (mean difference in SIDAS score, -6.5 points; 95% CI, -8.2 to -4.8 points; P < .001). Resolution of suicidality assessed by PHQ-9 item 9 occurred in 11 individuals (52%) with immediate testosterone commencement compared with 1 (5%) receiving standard care (P = .002). Seven individuals reported injection site pain/discomfort and 1 individual reported a transient headache 24 hours following intramuscular administration of testosterone undecanoate. No individual developed polycythemia.
Conclusions and relevance: In this open-label randomized clinical trial of testosterone therapy in transgender and gender-diverse adults, immediate testosterone compared with no treatment significantly reduced gender dysphoria, depression, and suicidality in transgender and gender-diverse individuals desiring testosterone therapy.
Trial registration: ANZCTR Identifier: ACTRN1262100016864.
Conflict of interest statement
References
-
- Maguen S, Shipherd JC. Suicide risk among transgender individuals. Psychol Sex. 2010;1(1):34-43.