Durvalumab Plus Concurrent Radiotherapy for Treatment of Locally Advanced Non-Small Cell Lung Cancer: The DOLPHIN Phase 2 Nonrandomized Controlled Trial
- PMID: 37676681
- PMCID: PMC10485744
- DOI: 10.1001/jamaoncol.2023.3309
Durvalumab Plus Concurrent Radiotherapy for Treatment of Locally Advanced Non-Small Cell Lung Cancer: The DOLPHIN Phase 2 Nonrandomized Controlled Trial
Abstract
Importance: Administration of durvalumab after concurrent chemoradiotherapy is the standard treatment of unresectable, locally advanced non-small cell lung cancer (NSCLC); however, 20% to 30% of patients do not receive durvalumab because of adverse events (AEs) during concurrent chemoradiotherapy. In addition, radiotherapy and immunotherapy have a synergistic effect.
Objective: To investigate the efficacy and safety of durvalumab immunotherapy plus concurrent radiotherapy followed by maintenance with durvalumab therapy for treatment of locally advanced NSCLC without chemotherapy.
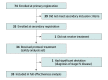
Design, setting, and participants: The multicenter, single-arm DOLPHIN (Phase II Study of Durvalumab [MEDI4736] Plus Concurrent Radiation Therapy in Advanced Localized NSCLC Patients) nonrandomized controlled trial was performed by 12 institutions in Japan from September 13, 2019, to May 31, 2022. Participants in the primary registration phase included 74 patients with programmed cell death ligand 1 (PD-L1)-positive, unresectable, locally advanced NSCLC. The current analyses were conducted from June 1, 2022, to October 31, 2022.
Interventions: Patients received radiotherapy (60 Gy) in combination with concurrent and maintenance durvalumab immunotherapy, 10 mg/kg every 2 weeks, for up to 1 year.
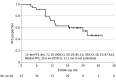
Main outcomes and measures: The primary end point of the rate of 12-month progression-free survival (PFS), as assessed by an independent central review, was estimated using the Kaplan-Meier method and evaluated with 90% CIs calculated using the Greenwood formula. The key secondary end points were PFS, objective response rate, treatment completion rate, and AEs.
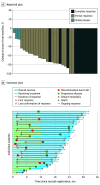
Results: Data from 35 patients (median [range] age, 72 [44-83] years; 31 [88.6%] men) were included in the full analysis set of the evaluable population. The 12-month PFS rate was 72.1% (90% CI, 59.1%-85.1%), and the median PFS was 25.6 months (95% CI, 13.1 months to not estimable) at a median follow-up of 22.8 months (range, 4.3-31.8 months). Scheduled radiation therapy was completed in 97.1% of patients. The confirmed objective response rate was 90.9% (95% CI, 75.7%-98.1%), and the treatment completion rate was 57.6% (95% CI, 39.2%-74.5%). Among 34 patients evaluated in the safety analysis set, AEs of grade 3 or 4 occurred in 18 patients (52.9%), and of grade 5 in 2 patients (5.9%). Pneumonitis or radiation pneumonitis of any grade occurred in 23 patients (67.6%), and of grades 3 or 4 in 4 patients (11.8%).
Conclusions and relevance: Findings from this phase 2 nonrandomized controlled trial indicate that durvalumab immunotherapy combined with curative radiotherapy for patients with PD-L1-positive, unresectable, locally advanced NSCLC is a promising treatment with tolerable AEs and is appropriate as a study treatment for phase 3 clinical trials.
Trial registration: Japan Registry of Clinical Trials ID: jRCT2080224763.
Conflict of interest statement
Figures
References
-
- National Cancer Institute Surveillance, Epidemiology and End Results Program. SEER cancer statistics review (CRS) 1975-2016. Updated April 9, 2020. Accessed July 14, 2023. (https://seer.cancer.gov/csr/1975_2016/.
-
- Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. doi:10.1016/j.jtho.2015.09.009 - DOI - PubMed
-
- Zenke Y, Tsuboi M, Chiba Y, et al. . Effect of second-generation vs third-generation chemotherapy regimens with thoracic radiotherapy on unresectable stage III non–small-cell lung cancer: 10-year follow-up of a WJTOG0105 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):904-909. doi:10.1001/jamaoncol.2021.0113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

