Master Transcription Factor Reprogramming Unleashes Selective Translation Promoting Castration Resistance and Immune Evasion in Lethal Prostate Cancer
- PMID: 37676710
- PMCID: PMC10714140
- DOI: 10.1158/2159-8290.CD-23-0306
Master Transcription Factor Reprogramming Unleashes Selective Translation Promoting Castration Resistance and Immune Evasion in Lethal Prostate Cancer
Abstract
Signaling rewiring allows tumors to survive therapy. Here we show that the decrease of the master regulator microphthalmia transcription factor (MITF) in lethal prostate cancer unleashes eukaryotic initiation factor 3B (eIF3B)-dependent translation reprogramming of key mRNAs conferring resistance to androgen deprivation therapy (ADT) and promoting immune evasion. Mechanistically, MITF represses through direct promoter binding eIF3B, which in turn regulates the translation of specific mRNAs. Genome-wide eIF3B enhanced cross-linking immunoprecipitation sequencing (eCLIP-seq) showed specialized binding to a UC-rich motif present in subsets of 5' untranslated regions. Indeed, translation of the androgen receptor and major histocompatibility complex I (MHC-I) through this motif is sensitive to eIF3B amount. Notably, pharmacologic targeting of eIF3B-dependent translation in preclinical models sensitizes prostate cancer to ADT and anti-PD-1 therapy. These findings uncover a hidden connection between transcriptional and translational rewiring promoting therapy-refractory lethal prostate cancer and provide a druggable mechanism that may transcend into effective combined therapeutic strategies.
Significance: Our study shows that specialized eIF3B-dependent translation of specific mRNAs released upon downregulation of the master transcription factor MITF confers castration resistance and immune evasion in lethal prostate cancer. Pharmacologic targeting of this mechanism delays castration resistance and increases immune-checkpoint efficacy. This article is featured in Selected Articles from This Issue, p. 2489.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

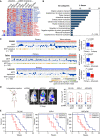


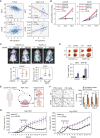

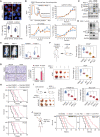
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol 2018;15:271–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

