Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury
- PMID: 37676731
- PMCID: PMC10629815
- DOI: 10.1172/jci.insight.173374
Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury
Abstract
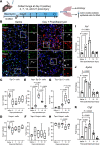
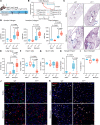
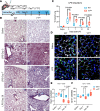
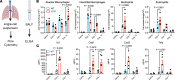
A hallmark of idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases is dysregulated repair of the alveolar epithelium. The Hippo pathway effector transcription factors YAP and TAZ are implicated as essential for type 1 and type 2 alveolar epithelial cell (AT1 and AT2) differentiation in the developing lung, yet aberrant activation of YAP/TAZ is a prominent feature of the dysregulated alveolar epithelium in IPF. In these studies, we sought to define the functional role of YAP/TAZ activity during alveolar regeneration. We demonstrated that Yap and Taz were normally activated in AT2 cells shortly after injury, and deletion of Yap/Taz in AT2 cells led to pathologic alveolar remodeling, failure of AT2-to-AT1 cell differentiation, increased collagen deposition, exaggerated neutrophilic inflammation, and increased mortality following injury induced by a single dose of bleomycin. Loss of Yap/Taz activity prior to an LPS injury prevented AT1 cell regeneration, led to intraalveolar collagen deposition, and resulted in persistent innate inflammation. These findings establish that AT2 cell Yap/Taz activity is essential for functional alveolar epithelial repair and prevention of fibrotic remodeling.
Keywords: Fibrosis; Mouse stem cells; Pulmonology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

